Chemistry:Cetirizine
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /sɛˈtɪrɪziːn/ |
| Trade names | Allacan, Piriteze, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a698026 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Well-absorbed (>70%)[1] |
| Protein binding | 88–96%[1] |
| Metabolism | Minimal (non-cytochrome P450-mediated)[3][2] |
| Onset of action | 20–42 minutes[2] |
| Elimination half-life | Mean: 8.3 hours[3][2] Range: 6.5–10 hours[4] |
| Duration of action | ≥24 hours[4] |
| Excretion | Urine: 70–85%[3] Feces: 10–13%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
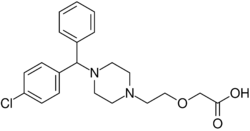
| Formula | C21H25ClN2O3 |
| Molar mass | 388.89 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Cetirizine is a second-generation antihistamine used to treat allergic rhinitis (hay fever), dermatitis, and urticaria (hives).[5] It is taken by mouth.[6] Effects generally begin within thirty minutes and last for about a day.[6] The degree of benefit is similar to other antihistamines such as diphenhydramine, which is a first-generation antihistamine.[6]
Common side effects include sleepiness, dry mouth, headache, and abdominal pain.[6] The degree of sleepiness that occurs is generally less than with first-generation antihistamines because second-generation antihistamines are more selective for the H1 receptor.[7][5] Compared to other second-generation anti-histamines, cetirizine can cause drowsiness.[7] Second-generation antihistamines that do not cause drowsiness are fexofenadine, and loratadine.[7] Therefore, it is important to avoid driving, and other dangerous scenarios while taking cetirizine.
Use in pregnancy appears safe, but use during breastfeeding is not recommended.[8] The medication works by blocking histamine H1 receptors, mostly outside the brain.[6]
Cetirizine can be used for paediatric patients. The main side effect to be cautious about is somnolence.[9]
It was patented in 1983[10][11] and came into medical use in 1987.[12] It is on the World Health Organization's List of Essential Medicines.[13] It is available as a generic medication.[5] In 2021, it was the 49th most commonly prescribed medication in the United States, with more than 13 million prescriptions.[14][15]
Medical uses
Allergies
Cetirizine's primary indication is for hay fever and other allergies. Because the symptoms of itching and redness in these conditions are caused by histamine acting on the H1 receptor, blocking those receptors temporarily relieves those symptoms.[16]
Cetirizine is also commonly prescribed to treat acute and (in particular cases) chronic urticaria, more efficiently than any other second-generation antihistamine.[16]
Available forms
Cetirizine is available over-the-counter in the US in the form of 5 and 10 mg tablets. A 20 mg strength is available by prescription only.[3] It is also available as a 1 mg/mL syrup for oral administration by prescription. In the UK, up to 30 tablets of 10 mg are on the general sales list (of pharmaceuticals) and can be purchased without a prescription and without pharmacist supervision.[17] The drug can be in the form of tablets, capsules or a syrup.[17]
Adverse effects
Commonly reported side effects of cetirizine include headache, dry mouth, drowsiness, and fatigue, while more serious, but rare, adverse effects reported include tachycardia and edema.[18]
Pruritus after discontinuation of cetirizine
Discontinuing cetirizine after prolonged use (typically, use beyond six months) may result in pruritus (generalized itchiness).[19][20][21]
The United States Food and Drug Administration (FDA) analyzed cases of pruritus after stopping cetirizine in the FDA Adverse Event Reporting System (FAERS) database and medical literature through 24 April 2017. Their report noted that some patients indicated the itchiness impacted their ability to work, sleep or perform normal daily activities.[22]
No specific schedule for weaning is currently provided in the drug information for cetirizine.[23]
Pharmacology
Pharmacodynamics
Cetirizine acts as a highly selective antagonist of the histamine H1 receptor.[3] The Ki values for the H1 receptor are approximately 6 nM for cetirizine, 3 nM for levocetirizine, and 100 nM for dextrocetirizine, indicating that the levorotatory enantiomer is the main active form.[3] Cetirizine has 600-fold or greater selectivity for the H1 receptor over a wide variety of other sites, including muscarinic acetylcholine, serotonin, dopamine, and α-adrenergic receptors, among many others.[3] The drug shows 20,000-fold or greater selectivity for the H1 receptor over the five muscarinic acetylcholine receptors, and hence does not exhibit anticholinergic effects.[24][25] It shows negligible inhibition of the hERG channel (IC50 > 30 μM)[26] and no cardiotoxicity has been observed with cetirizine at doses of up to 60 mg/day, six times the normal recommended dose[3] and the highest dose of cetirizine that has been studied in healthy subjects.[27]
Cetirizine crosses the blood–brain barrier only slightly, and for this reason, produces minimal sedation compared to many other antihistamines.[28] A positron emission tomography (PET) study found that brain occupancy of the H1 receptor was 12.6% for 10 mg cetirizine, 25.2% for 20 mg cetirizine, and 67.6% for 30 mg hydroxyzine.[29] (A 10 mg dose of cetirizine equals about a 30 mg dose of hydroxyzine in terms of peripheral antihistamine effect.)[30] PET studies with antihistamines have found that brain H1 receptor occupancy of more than 50% is associated with a high prevalence of somnolence and cognitive decline, whereas brain H1 receptor occupancy of less than 20% is considered to be non-sedative.[31] In accordance, H1 receptor occupancy correlated well with subjective sleepiness for 30 mg hydroxyzine but there was no correlation for 10 or 20 mg cetirizine.[29] As such, brain penetration and brain H1 receptor occupancy by cetirizine are dose-dependent, and in accordance, while cetirizine at doses of 5 to 10 mg have been reported to be non-sedating or mildly sedating, a higher dose of 20 mg has been found to induce significant drowsiness in other studies.[29]
Cetirizine also shows anti-inflammatory properties independent of H1 receptors.[32] The effect is exhibited through suppression of the NF-κB pathway, and by regulating the release of cytokines and chemokines, thereby regulating the recruitment of inflammatory cells.[33][34][35][36][37] It has been shown to inhibit eosinophil chemotaxis and LTB4 release.[38] At a dosage of 20 mg, Boone et al. found that it inhibited the expression of VCAM-1 in patients with atopic dermatitis.[38]
Pharmacokinetics
Absorption
Cetirizine is rapidly and extensively absorbed upon oral administration in tablet or syrup form.[3] The oral bioavailability of cetirizine is at least 70% and of levocetirizine is at least 85%.[1] The Tmax of cetirizine is approximately 1.0 hour regardless of formulation.[2] The pharmacokinetics of cetirizine have been found to increase linearly with dose across a range of 5 to 60 mg.[3] Its Cmax following a single dose has been found to be 257 ng/mL for 10 mg and 580 ng/mL for 20 mg.[2] Food has no effect on the bioavailability of cetirizine but has been found to delay the Tmax by 1.7 hours (i.e., to approximately 2.7 hours) and to decrease the Cmax by 23%.[3][2][39] Similar findings were reported for levocetirizine, which had its Tmax delayed by 1.25 hours and its Cmax decreased by about 36% when administered with a high-fat meal.[39] Steady-state levels of cetirizine occur within 3 days and there is no accumulation of the drug with chronic administration.[2] Following once-daily administration of 10 mg cetirizine for ten days, the mean Cmax was 311 ng/mL.[40]
Distribution
The mean plasma protein binding of cetirizine has been found to be 93 to 96% across a range of 25 to 1,000 ng/mL independent of concentration.[2] Plasma protein binding of 88 to 96% has also been reported across multiple studies.[1] The drug is bound to albumin with high affinity, while α1-acid glycoprotein and lipoproteins contribute much less to total plasma protein binding.[1] The unbound or free fraction of levocetirizine has been reported to be 8%.[1] The true volume of distribution of cetirizine is unknown but is estimated to be 0.3 to 0.45 L/kg.[3][1] Cetirizine poorly and slowly crosses the blood–brain barrier, which is thought to be due to its chemical properties and its activity as a P-glycoprotein substrate.[41][1][42]
Metabolism
Cetirizine does not undergo extensive metabolism.[3] It is notably not metabolized by the cytochrome P450 system.[43] Because of this, it does not interact significantly with drugs that inhibit or induce cytochrome P450 enzymes such as theophylline, erythromycin, clarithromycin, cimetidine, or alcohol.[3] While cetirizine does not undergo extensive metabolism or metabolism by the cytochrome P450 enzyme, it does undergo some metabolism by other means, the metabolic pathways of which include oxidation and conjugation.[3][2] Plasma radioactivity attributed to unchanged cetirizine is more than 90% at 2 hours, 80% at 10 hours, and 70% at 24 hours, indicating limited and slow metabolism.[2] The enzymes responsible for transformation of cetirizine have not been identified.[3]
Elimination
Cetirizine is eliminated approximately 70 to 85% in the urine and 10 to 13% in the feces.[3] About 50 or 60% of cetirizine eliminated in the urine is unchanged.[3][2] It is eliminated in the urine via an active transport mechanism.[2] The elimination half-life of cetirizine ranges from 6.5 to 10 hours in healthy adults, with a mean across studies of approximately 8.3 hours.[3][2] Its duration of action is at least 24 hours.[2] The elimination half-life of cetirizine is increased in the elderly (to 12 hours), in hepatic impairment (to 14 hours), and in renal impairment (to 20 hours).[2]
Chemistry
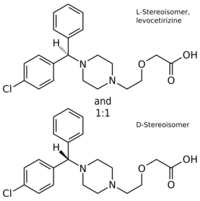
Cetirizine contains L- and D-stereoisomers. Chemically, levocetirizine is the active L-enantiomer of cetirizine. The drug is a member of the diphenylmethylpiperazine group of antihistamines. Analogues include cyclizine and hydroxyzine.[44]
Synthesis
 Cetirizine synthesis:[10]
Cetirizine synthesis:[10]
The 1-(4-chlorophenylmethyl)-piperazine is alkylated with methyl (2-chloroethoxy)-acetate in the presence of sodium carbonate and xylene solvent to produce the Sn2 substitution product in 28% yield. Saponification of the acetate ester is done by refluxing with potassium hydroxide in absolute ethanol to afford a 56% yield of the potassium salt intermediate. This is then hydrolyzed with aqueous HCl and extracted to give an 81% yield of the carboxylic acid product.[45]
Availability
Cetirizine is available without a prescription.[46] In some countries it is available over-the-counter only in packages containing seven or ten 10 mg doses.[47][48]
Cetirizine is available as a combination medication with pseudoephedrine, a decongestant.[49] The combination is often marketed using the same brand name as the cetirizine with a "-D" suffix (for example, Zyrtec-D).[50][51]
Cetirizine is marketed under the brand names Alatrol, Alerid, Allacan, Allercet, Alzene, Cerchio, Cetirin, Cetizin, Cetriz, Cetzine, Cezin, Cetgel, Cirrus, Histec, Histazine, Humex, Letizen, Okacet (Cipla), Piriteze, Reactine, Razene, Rigix, Sensahist (Oethmann, South Africa), Triz, Zetop, Zirtec, Zirtek, Zodac, Zyllergy, Zynor, Zyrlek, and Zyrtec (Johnson & Johnson), among others.[52][53][failed verification]
References
- ↑ Jump up to: 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Physicochemical, pharmacological and pharmacokinetic properties of the zwitterionic antihistamines cetirizine and levocetirizine". Current Medicinal Chemistry 15 (21): 2173–2191. 2008. doi:10.2174/092986708785747625. PMID 18781943.
- ↑ Jump up to: 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 "Clinical pharmacology of new histamine H1 receptor antagonists". Clinical Pharmacokinetics 36 (5): 329–352. May 1999. doi:10.2165/00003088-199936050-00003. PMID 10384858.
- ↑ Jump up to: 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 "Review of cetirizine hydrochloride for the treatment of allergic disorders". Expert Opinion on Pharmacotherapy 5 (1): 125–135. January 2004. doi:10.1517/14656566.5.1.125. PMID 14680442.
- ↑ Jump up to: 4.0 4.1 "Comparative pharmacology of H1 antihistamines: clinical relevance". The American Journal of Medicine 113 (Suppl 9A): 38S–46S. December 2002. doi:10.1016/s0002-9343(02)01436-5. PMID 12517581.
- ↑ Jump up to: 5.0 5.1 5.2 British national formulary : BNF 76 (76 ed.). Pharmaceutical Press. 2018. pp. 279. ISBN 9780857113382.
- ↑ Jump up to: 6.0 6.1 6.2 6.3 6.4 "Cetirizine Hydrochloride Monograph for Professionals". American Society of Health-System Pharmacists. https://www.drugs.com/monograph/cetirizine-hydrochloride.html.
- ↑ Jump up to: 7.0 7.1 7.2 "Second-generation antihistamines: a comparative review". Drugs 57 (1): 31–47. January 1999. doi:10.2165/00003495-199957010-00004. PMID 9951950.
- ↑ "Cetirizine Pregnancy and Breastfeeding Warnings" (in en). https://www.drugs.com/pregnancy/cetirizine.html.
- ↑ "Cetirizine for the treatment of allergic diseases in children: A systematic review and meta-analysis". Frontiers in Pediatrics 10: 940213. 25 August 2022. doi:10.3389/fped.2022.940213. PMID 36090559.
- ↑ Jump up to: 10.0 10.1 Baltes E, De Lannoy J, Rodriguez L, "2-[4-(Diphenylmethyl)-1-piperazinyl]-acetic acids and their amides", US patent 4525358, issued 25 June 1985, assigned to UCB Pharmaceuticals, Inc.
- ↑ , Eugene; Jean de Lannoy & Ludovic Rodriguez"2-[4-(Diphenylmethyl)-1-piperazinyl]-acetic acids and their amides" patent US4525358A, issued 1985-06-25
- ↑ (in en) Analogue-based Drug Discovery. John Wiley & Sons. 2006. p. 549. ISBN 9783527607495. https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA549. Retrieved 19 September 2020.
- ↑ World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. 2021. WHO/MHP/HPS/EML/2021.02.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Cetirizine - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Cetirizine.
- ↑ Jump up to: 16.0 16.1 Rang and Dale's pharmacology (Eighth ed.). [United Kingdom]. 21 January 2015. pp. 332. ISBN 978-0-7020-5362-7. OCLC 903083639. https://www.worldcat.org/oclc/903083639.
- ↑ Jump up to: 17.0 17.1 "CETIRIZINE HYDROCHLORIDE". https://bnf.nice.org.uk/drug/cetirizine-hydrochloride.html.
- ↑ "Zyrtec Side Effects". https://www.drugs.com/sfx/zyrtec-side-effects.html.
- ↑ "Unbearable Pruritus After Withdrawal of (Levo)cetirizine". Drug Safety: Case Reports 3 (1): 16. December 2016. doi:10.1007/s40800-016-0041-9. PMID 27889900.
- ↑ "Cetirizine (Zyrtec) Withdrawal & Unbearable Itching". https://www.peoplespharmacy.com/2013/05/06/cetirizine-zyrtec-withdrawal-unbearable-itching/.
- ↑ "addicted to zyrtec?". http://www.medhelp.org/posts/Allergy/addicted-to-zyrtec/show/600862.
- ↑ "Pruritus after discontinuation of cetirizine". Therapeutic Advances in Drug Safety 10: 2042098619859996. 2019. doi:10.1177/2042098619859996. PMID 31308927.
- ↑ "Did you know stopping Zyrtec can cause withdrawal?" (in en-US). 18 May 2023. https://www.singlecare.com/blog/zyrtec-withdrawal/.
- ↑ "The clinical use of cetirizine in the treatment of allergic rhinitis". Pharmacology 92 (1–2): 14–25. 2013. doi:10.1159/000351843. PMID 23867423.
- ↑ "Comparative anticholinergic activities of 10 histamine H1 receptor antagonists in two functional models". European Journal of Pharmacology 506 (3): 257–264. January 2005. doi:10.1016/j.ejphar.2004.11.006. PMID 15627436.
- ↑ "Molecular basis for the lack of HERG K+ channel block-related cardiotoxicity by the H1 receptor blocker cetirizine compared with other second-generation antihistamines". Molecular Pharmacology 54 (1): 113–121. July 1998. doi:10.1124/mol.54.1.113. PMID 9658196.
- ↑ "Levocetirizine does not prolong the QT/QTc interval in healthy subjects: results from a thorough QT study". European Journal of Clinical Pharmacology 63 (11): 1011–1017. November 2007. doi:10.1007/s00228-007-0366-5. PMID 17891537. "The equivalent dose of 60 mg cetirizine is also the highest dose ever administered in healthy subjects [13].".
- ↑ "Brain distribution of cetirizine enantiomers: comparison of three different tissue-to-plasma partition coefficients: K(p), K(p,u), and K(p,uu)". Drug Metabolism and Disposition 34 (2): 318–323. February 2006. doi:10.1124/dmd.105.007211. PMID 16303872.
- ↑ Jump up to: 29.0 29.1 29.2 "Dose dependency of brain histamine H(1) receptor occupancy following oral administration of cetirizine hydrochloride measured using PET with [11C]doxepin". Human Psychopharmacology 24 (7): 540–548. October 2009. doi:10.1002/hup.1051. PMID 19697300.
- ↑ "Effectiveness and safety of antihistamines up to fourfold or higher in treatment of chronic spontaneous urticaria". Clinical and Translational Allergy 7: 4. 2017. doi:10.1186/s13601-017-0141-3. PMID 28289538. "[...] 30 mg of hydroxyzine equals about 10 mg cetirizine [11] [...]".
- ↑ "The physiological and pathophysiological roles of neuronal histamine: an insight from human positron emission tomography studies". Pharmacology & Therapeutics 113 (1): 1–15. January 2007. doi:10.1016/j.pharmthera.2006.06.008. PMID 16890992.
- ↑ "Cetirizine exerts anti-inflammatory effects on human neutrophils". International Archives of Allergy and Immunology 110 (1): 52–56. May 1996. doi:10.1159/000237310. PMID 8645978.
- ↑ "Efficacy and tolerability of newer antihistamines in the treatment of allergic conjunctivitis". Drugs 65 (2): 215–228. 2005. doi:10.2165/00003495-200565020-00004. PMID 15631542.
- ↑ "The anti-inflammatory effects of cetirizine". Clinical and Experimental Allergy 24 (1): 81–85. January 1994. doi:10.1111/j.1365-2222.1994.tb00921.x. PMID 8156449.
- ↑ "Anti-inflammatory activity of H1-receptor antagonists: review of recent experimental research". Current Medical Research and Opinion 20 (1): 73–81. January 2004. doi:10.1185/030079903125002586. PMID 14741075.
- ↑ "Antihistamines: do they work? Further well-controlled trials involving larger samples are needed". Allergy 59 (Suppl 78): 74–77. August 2004. doi:10.1111/j.1398-9995.2004.00573.x. PMID 15245363.
- ↑ "Antiinflammatory properties of cetirizine in a human contact dermatitis model. Clinical evaluation of patch tests is not hampered by antihistamines". Acta Dermato-Venereologica 78 (3): 194–197. May 1998. doi:10.1080/000155598441512. PMID 9602225.
- ↑ Jump up to: 38.0 38.1 "Adhesion molecule profiles in atopic dermatitis vs. allergic contact dermatitis: pharmacological modulation by cetirizine". Journal of the European Academy of Dermatology and Venereology 14 (4): 263–266. July 2000. doi:10.1046/j.1468-3083.2000.00017.x. PMID 11204513.
- ↑ Jump up to: 39.0 39.1 "Second generation H1 - antihistamines interaction with food and alcohol-A systematic review". Biomedicine & Pharmacotherapy 93: 27–39. September 2017. doi:10.1016/j.biopha.2017.06.008. PMID 28622592.
- ↑ "Zyrtec prescribing information". May 2006. http://www.pfizer.com/files/products/uspi_zyrtec.pdf.
- ↑ "Why are second-generation H1-antihistamines minimally sedating?". European Journal of Pharmacology 765: 100–106. October 2015. doi:10.1016/j.ejphar.2015.08.016. PMID 26291661.
- ↑ "The role of P-glycoprotein in CNS antihistamine effects". Psychopharmacology 229 (1): 9–19. September 2013. doi:10.1007/s00213-013-3075-z. PMID 23564211.
- ↑ Allergy and Asthma: Practical Diagnosis and Management. Springer. 2 June 2016. pp. 574–. ISBN 978-3-319-30835-7. https://books.google.com/books?id=spdPDAAAQBAJ&pg=PA574.
- ↑ "Cetirizine". PubChem. U.S. Naionatl Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/2678.
- ↑ "New Manufacturing Procedure of Cetirizine" (in en). Organic Process Research & Development 16 (7): 1279–1282. 20 July 2012. doi:10.1021/op300009y. ISSN 1083-6160.
- ↑ "Cetirizine: Clinical Review". U.S. Food and Drug Administration. 11 September 2016. https://www.fda.gov/media/105988/download.
- ↑ "Evaluation of cetirizine in patients with allergic rhinitis and perennial asthma". Annals of Allergy, Asthma & Immunology 76 (5): 440–446. May 1996. doi:10.1016/s1081-1206(10)63461-8. PMID 8630718.
- ↑ "Assessment of the efficacy and safety of three dose levels of cetirizine given once daily in children with perennial allergic rhinitis". Allergy 49 (8): 598–604. September 1994. doi:10.1111/j.1398-9995.1994.tb00125.x. PMID 7653736.
- ↑ "Cetirizine/pseudoephedrine". Drugs 61 (15): 2231–2240. 2001. doi:10.2165/00003495-200161150-00009. PMID 11772135.
- ↑ "Comparison of cetirizine-pseudoephedrine and placebo in patients with seasonal allergic rhinitis and concomitant mild-to-moderate asthma: randomized, double-blind study". Annals of Allergy, Asthma & Immunology 97 (3): 389–396. September 2006. doi:10.1016/S1081-1206(10)60806-X. PMID 17042147.
- ↑ "Antihistamine/Decongestant Combination (Oral Route) Description and Brand Names". 7 February 2023. https://www.mayoclinic.org/drugs-supplements/antihistamine-decongestant-combination-oral-route/description/drg-20069883.
- ↑ "Cetirizine: antihistamine that relieves allergy symptoms" (in en). 30 October 2018. https://www.nhs.uk/medicines/cetirizine/.
- ↑ "Protriptyline". AHFS Patient Medication Information [Internet]. Bethesda (MD): American Society of Health-System Pharmacists, Inc.. 2019. https://medlineplus.gov/druginfo/meds/a698026.html#brand-name-1.
 |