Chemistry:Uvaricin
From HandWiki
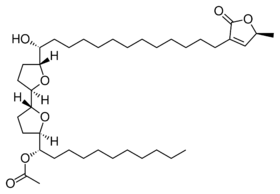
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S)-1-[(2R,2′R,5R,5′R)-5′-{(1R)-1-Hydroxy-13-[(5S)-5-methyl-2-oxo-2,5-dihydrofuran-3-yl]tridecyl}[2,2′-bioxolan]-5-yl]undecyl acetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C39H68O7 | |
| Molar mass | 648.966 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Uvaricin is a bis(tetrahydrofuranoid) fatty acid lactone that was first isolated in 1982 from the roots of the Annonaceae Uvaria acuminata.[1] Uvaricin was the first known example in a class of compounds known as acetogenins. Acetogenins, which are found in plants of the family Annonaceae, seem to kill cells by inhibiting NADH dehydrogenase in the mitochondrion.[2] A method to synthesize uvaricin was first published in 1998,[3] and an improved stereoselective synthesis published in 2001.[4]
References
- ↑ Jolad, S. D.; Hoffmann, J. J.; Schram, K. H.; Cole, J. R.; Tempesta, M. S.; Kriek, G. R.; Bates, R. B. (1982). "Uvaricin, a New Antitumor Agent from Uvaria accuminata (Annonaceae)". Journal of Organic Chemistry 47 (16): 3151–3153. doi:10.1021/jo00137a024.
- ↑ Zafra-Polo, M. C.; González, M. C.; Estornell, E.; Sahpaz, S.; Cortes, D. (1996). "Acetogenins from Annonaceae, Inhibitors of Mitochondrial Complex I". Phytochemistry 42 (2): 253–271. doi:10.1016/0031-9422(95)00836-5. PMID 8688168.
- ↑ Yazbak, A.; Sinha, S. C.; Keinan, E. (1998). "Total Synthesis of Uvaricin". Journal of Organic Chemistry 63 (17): 5863–5868. doi:10.1021/jo980453a. PMID 11672188. http://www.technion.ac.il/~keinanj/pub/92.pdf. Retrieved 2008-07-14.
- ↑ Burke, S. D.; Jiang, L. (2001). "Formal Synthesis of Uvaricin via Palladium-Mediated Double Cyclization". Organic Letters 3 (12): 1953–1956. doi:10.1021/ol0160304. PMID 11405753.
 |
