Chemistry:Vanadate
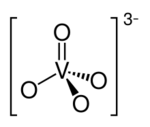
In chemistry, a vanadate is an anionic coordination complex of vanadium. Often vanadate refers to oxoanions of vanadium, most of which exist in its highest oxidation state of +5. The complexes [V(CN)
6]3− and [V
2Cl
9]3− are referred to as hexacyanovanadate(III) and nonachlorodivanadate(III), respectively.
A simple vanadate ion is the tetrahedral orthovanadate anion, VO3−
4 (which is also called vanadate(V)), which is present in e.g. sodium orthovanadate and in solutions of V
2O
5 in strong base (pH > 13[1]). Conventionally this ion is represented with a single double bond, however this is a resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms.
Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions.[2] There are also vanadates, such as rhodium vanadate, RhVO
4, which has a statistical rutile structure where the Rh3+ and V5+ ions randomly occupy the Ti4+ positions in the rutile lattice,[3] that do not contain a lattice of cations and balancing vanadate anions but are mixed oxides.
In chemical nomenclature when vanadate forms part of the name, it indicates that the compound contains an anion with a central vanadium atom, e.g. ammonium hexafluorovanadate is a common name for the compound [NH
4]
3[VF
6] with the IUPAC name of ammonium hexafluoridovanadate(III).
Examples of oxovanadate ions
Some examples of discrete ions are
- VO3−
4 "orthovanadate", tetrahedral.[2] - V
2O4−
7 "pyrovanadate", corner-shared VO
4 tetrahedra, similar to the dichromate ion[2] - V
3O3−
9, cyclic with corner-shared VO
4 tetrahedra[4] - V
4O4−
12, cyclic with corner-shared VO
4 tetrahedra[5] - V
5O3−
14, corner shared VO
4 tetrahedra[6] - V
6O6−
18, ring.[7] - V
10O6−
28 "decavanadate", edge- and corner-shared VO
6 octahedra[2] - V
12O4−
32 - V
13O3−
34, fused VO
6 octahedra [8] - V
18O12−
42[9]
Some examples of polymeric "infinite" ions are
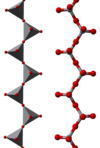 |
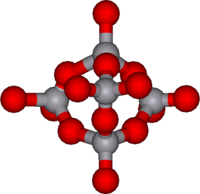 |
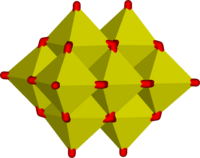 |
metavanadate chains
|
V
5O 14 |
decavanadate ion
|
In these ions vanadium exhibits tetrahedral, square pyramidal and octahedral coordination. In this respect vanadium shows similarities to tungstate and molybdate, whereas chromium however has a more limited range of ions.
Aqueous solutions
Dissolution of vanadium pentoxide in strongly basic aqueous solution gives the colourless VO3−
4 ion. On acidification, this solution's colour gradually darkens through orange to red at around pH 7. Brown hydrated V2O5 precipitates around pH 2, redissolving to form a light yellow solution containing the [VO
2(H
2O)
4]+
ion. The number and identity of the oxyanions that exist between pH 13 and 2 depend on pH as well as concentration. For example, protonation of vanadate initiates a series of condensations to produce polyoxovanadate ions:[2]
- pH 9–12: HVO2−
4, V
2O4−
7 - pH 4–9: H
2VO−
4, V
4O4−
12, HV
10O5−
28 - pH 2–4: H
3VO
4, H
2V
10O4−
28
Pharmacological properties
Vanadate is a potent inhibitor of certain plasma membrane ATPases, such as Na+/K+-ATPase and Ca2+-ATPase (PMCA). Acting as a transition-state analog of phosphate, vanadate undergoes nucleophillic attack by water during phosphoryl transfer, essentially "trapping" P-type ATPases in their phosphorylated E2 state. [11][12] It also inhibits skeletal muscle actomyosin MgATPase activity[13] and calcium activated force generation by actomyosin in the intact skeletal muscle contractile apparatus.[14] However, it does not inhibit other ATPases, such as SERCA (sarco/endoplasmic reticulum Ca2+-ATPase) or mitochondrial ATPase.[15][16][17]
References
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Hamilton E. E.; Fanwick P.E.; Wilker J.J. (2002). "The Elusive Vanadate (V3O9)3−: Isolation, Crystal Structure, and Nonaqueous Solution Behavior". J. Am. Chem. Soc. 124 (1): 78–82. doi:10.1021/ja010820r. PMID 11772064. https://figshare.com/articles/The_Elusive_Vanadate_V_sub_3_sub_O_sub_9_sub_sup_3-_sup_Isolation_Crystal_Structure_and_Nonaqueous_Solution_Behavior/3634506.
- ↑ G.-Y. Yang, D.-W. Gao, Y. Chen, J.-Q. Xu, Q.-X. Zeng, H.-R. Sun, Z.-W. Pei, Q. Su, Y. Xing, Y.-H. Ling and H.-Q. Jia (1998). "[Ni(C10H8N2)3]2[V4O12]·11H2O". Acta Crystallographica C 54 (5): 616–618. doi:10.1107/S0108270197018751. Bibcode: 1998AcCrC..54..616Y.
- ↑ V. W. Day; Walter G. Klemperer; O. M. Yaghi (1989). "A new structure type in polyoxoanion chemistry: synthesis and structure of the V5O3−14 anion". J. Am. Chem. Soc. 111 (12): 4518. doi:10.1021/ja00194a068.
- ↑ Guang-Chuan Ou.; Long Jiang; Xiao-Long Feng; Tong-Bu Lu (2009). "Vanadium polyoxoanion-bridged macrocyclic metal complexes: from one-dimensional to three-dimensional structures". Dalton Transactions 1 (1): 71–76. doi:10.1039/B810802A. PMID 19081973.
- ↑ Hou D.; Hagen K.D.; Hill C.L. (1992). "Tridecavanadate, [V13O34]3−, a new high-potential isopolyvanadate". J. Am. Chem. Soc. 114 (14): 5864. doi:10.1021/ja00040a061.
- ↑ Müller A.; Sessoli R.; Krickemeyer E.; Bögge H.; Meyer J.; Gatteschi D.; Pardi L.; Westphal J. et al. (1997). "Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host–Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies". Inorg. Chem. 36 (23): 5239. doi:10.1021/ic9703641.
- ↑ Jouanneau, S.; Verbaere, A.; Guyomard, D. (2003). "On a new calcium vanadate: synthesis, structure and Li insertion behaviour". Journal of Solid State Chemistry 172 (1): 116–122. doi:10.1016/S0022-4596(02)00164-0. Bibcode: 2003JSSCh.172..116J.
- ↑ Kühlbrandt, Werner (April 2004). "Biology, structure and mechanism of P-type ATPases". Nature Reviews. Molecular Cell Biology 5 (4): 282–295. doi:10.1038/nrm1354. ISSN 1471-0072. PMID 15071553.
- ↑ Davies, Douglas R.; Hol, Wim G.J. (2004-11-19). "The power of vanadate in crystallographic investigations of phosphoryl transfer enzymes" (in en). FEBS Letters 577 (3): 315–321. doi:10.1016/j.febslet.2004.10.022. ISSN 0014-5793. PMID 15556602.
- ↑ Goodno, C.C.; Taylor, E.W. (1982). "Inhibition of actomyosin ATPase by vanadate". Proceedings of the National Academy of Sciences USA 79 (1): 21–25. doi:10.1073/pnas.79.1.21. PMID 6459580. Bibcode: 1982PNAS...79...21G.
- ↑ Wilson, G.J.; Shull, S.E.; Cooke, R. (1995). "Inhibition of muscle force by vanadate". Biophysical Journal 68 (1): 216–226. doi:10.1016/S0006-3495(95)80177-3. PMID 7711244. Bibcode: 1995BpJ....68..216W.
- ↑ Luo D.; Nakazawa M.; Yoshida Y.; Cai J.; Imai S. (2000). "Effects of three different Ca2+ pump ATPase inhibitors on evoked contractions in rabbit aorta and activities of Ca2+ pump ATPases in porcine aorta". General Pharmacology: The Vascular System 34 (3): 211–220. doi:10.1016/S0306-3623(00)00064-1. PMID 11120383.
- ↑ Bowman B.J.; Slayman C.W. (1979). "The Effects of Vanadate on the Plasma Membrane ATPase of Neurospora crassa". Journal of Biological Chemistry 254 (8): 2928–2934. doi:10.1016/S0021-9258(17)30163-1. PMID 155060.
- ↑ Aureliano, Manuel; Crans, Debbie C. (2009). "Decavanadate (V10O6−28) and oxovanadates: Oxometalates with many biological activities". Journal of Inorganic Biochemistry 103 (4): 536–546. doi:10.1016/j.jinorgbio.2008.11.010. ISSN 0162-0134. PMID 19110314. https://www.sciencedirect.com/science/article/pii/S0162013408002882.
 |
