Chemistry:Vanadium tetrachloride
|
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
Vanadium tetrachloride
Vanadium(IV) chloride | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| VCl4 | |||
| Molar mass | 192.75 g/mol | ||
| Appearance | bright red liquid, moisture sensitive | ||
| Odor | pungent | ||
| Density | 1.816 g/cm3, liquid | ||
| Melting point | −24.5 °C (−12.1 °F; 248.7 K) | ||
| Boiling point | 148 °C (298 °F; 421 K) | ||
| decomposes | |||
| Solubility | soluble in CH2Cl2 | ||
| Vapor pressure | 7.9 Pa | ||
| +1130.0·10−6 cm3/mol | |||
| Structure | |||
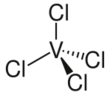
| tetrahedral | |||
| 0 D | |||
| Hazards | |||
| Main hazards | toxic; oxidizer; hydrolyzes to release HCl | ||
| NFPA 704 (fire diamond) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
160 mg/kg (rat, oral) | ||
| Related compounds | |||
Other anions
|
vanadium tetrafluoride, vanadium disulfide, vanadium tetrabromide | ||
Other cations
|
titanium tetrachloride, chromium tetrachloride, niobium tetrachloride, tantalum tetrachloride | ||
Related compounds
|
vanadium trichloride | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Vanadium tetrachloride is the inorganic compound with the formula VCl4. This reddish-brown liquid serves as a useful reagent for the preparation of other vanadium compounds.
Synthesis, bonding, basic properties
With one more valence electron than diamagnetic TiCl4, VCl4 is a paramagnetic liquid. It is one of only a few paramagnetic compounds that is liquid at room temperature.
VCl4 is prepared by chlorination of vanadium metal. VCl5 does not form in this reaction; Cl2 lacks the oxidizing power to attack VCl4. VCl5 can however be prepared indirectly from VF5 at −78 °C.[1]
Reactions
Consistent with its high oxidizing power, VCl4 reacts with HBr at -50 °C to produce VBr3. The reaction proceeds via VBr4, which releases Br2 during warming to room temperature.[2]
- 2 VCl4 + 8 HBr → 2 VBr3 + 8 HCl + Br2
VCl4 forms adducts with many donor ligands, for example, VCl4(THF)2.
It is the precursor to vanadocene dichloride.
Organic chemistry
In organic synthesis, VCl4 is used for the oxidative coupling of phenols. For example, it converts phenol into a mixture of 4,4'-, 2,4'-, and 2,2'-biphenols:[3]
- 2 C6H5OH + 2 VCl4 → HOC6H4–C6H4OH + 2 VCl3 + 2 HCl
Applications
VCl4 is a catalyst for the polymerization of alkenes, especially those useful in the rubber industry. The underlying technology is related to Ziegler–Natta catalysis, which involves the intermediacy of vanadium alkyls.
Safety considerations
VCl4 is a volatile, aggressive oxidant that readily hydrolyzes to release HCl.
References
- ↑ Tamadon, Farhad; Seppelt, Konrad (2013). "The Elusive Halides VCl5, MoCl6, and ReCl6". Angew. Chem. Int. Ed. 52 (2): 767–769. doi:10.1002/anie.201207552. PMID 23172658.
- ↑ Calderazzo, F.; Maichle-Mössmer, C.; G., Pampaloni; J., Strähle (1993). "Low-temperature Syntheses of Vanadium(III) and Molybdenum(IV) Bromides by Halide Exchange". Dalton Transactions (5): 655–8. doi:10.1039/DT9930000655.
- ↑ O’Brien, M. K.; Vanasse, B. (2001). "Vanadium(IV) Chloride". in Paquette, L.. Encyclopedia of Reagents for Organic Synthesis. New York, NY: J. Wiley & Sons. doi:10.1002/047084289X.rv001. ISBN 0471936235.
 |