Chemistry:Vanishing valentine experiment
File:Vanishing Valentine.webm The vanishing valentine experiment is a type of chemical reaction related to the blue bottle experiment. This reaction occurs when water, glucose, sodium hydroxide, and resazurin is mixed in a flask. When the solution is shaken, it turns from light blue to a redish color. The solution turns back to a light blue after being left to stand for a while. This reaction can be repeated several times.[1]
After mixing all the components, shake the bottle and the color will turn to red or pink depend on the amount of resazurin in the solution. More resazurin will result in more time needed for the solution to turn back the color and the intensity of the red color.
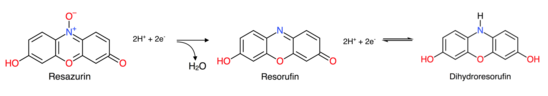
Chemical reaction
Glucose reduces resazurin to resorufin which will then be reduced again to a colorless compound dihydroresorufin. When dihydroresorufin is shaken, it is oxidized back to resorufin because when shook, the oxygen in the bottle will oxidize dihydroresorufin and make it back into resorufin.[2]
Pattern formation
Vanishing Valentine pattern formation is the new modification to the original blue bottle experiment which has a similar mechanism.[3] This experiment was developed by Steven Engerer and Gilbert Cook, inspired by Campbell's work from 1963. Later in 1994, Cook made changes to the classic blue bottle experiment in his article called "Blue Bottle Experiment Revisited".[4] The possible application of this experiment is to identify oxygen level of intelligence food packaging and oxygen scavenger for super-resolution imaging.[3] Instead of using methylene blue (blue bottle experiment), Vanishing Valentine uses resazurin that the solution will turn into colorless/fluorescent red.
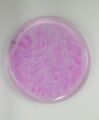
During the experiment, the solution will be tested on a thin petri dish to be easily observed. With the mixing of sodium hydroxide, glucose and resazurin the color of the solution will turn to be light pink. Then, stir gently until the whole solution turns bright pink. After leaving the solution for a few minutes to oxidized, the bright pink colour will slowly fade into light pink.[5] For a certain time, the patterns will start to develop due to different densities of glucose and the oxidized product.[4] In the beginning of pattern formations, the patterns will start developing with a ring on the edge of the petri dish. Then, numerous dot-like pattern will appear, furthermore these dotted pattern will start to move in spiral or current-like movement. In addition, with more trial of stirring, the solution patterns will be slightly different. In other words, the first trial will acquire the clearest dots-like pattern.[3]
Additional media
See also
References
- ↑ "Vanishing Valentine Chemistry Demonstration". http://chemistry.about.com/od/valentinesdaychemistry/a/Vanishing-Valentine-Chemistry-Demonstration.html. Retrieved 13 November 2015.
- ↑ "The Vanishing Vaentine". http://www.flinnsci.com/documents/demopdfs/chemistry/cf0667.00.pdf. Retrieved 13 November 2015.
- ↑ 3.0 3.1 3.2 Rajchakit, Urawadee; Limpanuparb, Taweetham (2016-01-01). "The Blue Bottle Experiment" (in th). Thai Journal of Science and Technology 24 (1): 1–11. ISSN 0858-4435. https://www.tci-thaijo.org/index.php/tstj/article/view/42576.
- ↑ 4.0 4.1 Limpanuparb, Taweetham; Hsu, Suphattra (2015-04-10). "The Colorful Chemical Bottle Experiment Kit: From School Laboratory To Public Demonstration". arXiv:1504.02604.
- ↑ The Vanishing Valentine. Batavia, IL: Flint Scientific. 2010. p. 2. http://www.flinnsci.com/documents/demopdfs/chemistry/cf0667.00.pdf. Retrieved 11 February 2016.