Chemistry:Vasicinone
From HandWiki
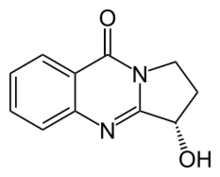
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1S)-1-Hydroxy-2,3-dihydropyrrolo[2,1-b]quinazolin-5(1H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H10N2O2 | |
| Molar mass | 202.213 g·mol−1 |
| Density | 1.5 g/cm3 |
| Melting point | 200 to 202 °C (392 to 396 °F; 473 to 475 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vasicinone is a quinazoline alkaloid. It shows bronchodilator action in vitro[1] but bronchoconstrictor action in vivo.[2] Vasicinone was shown to have an antianaphylactic action.[3] It has been found within Peganum harmala.[4]
Vasicinone has also been studied in combination with the related alkaloid vasicine. Both the alkaloids in combination (1:1) showed pronounced bronchodilatory activity in vivo and in vitro.[5] Both alkaloids are also respiratory stimulants.[5] Vasicine has a cardiac–depressant effect, while vasicinone is a weak cardiac stimulant; the effect can be normalized by combining the alkaloids.[5][3] Vasicine is reported to have a uterine stimulant effect.[3]
References
- ↑ Amin, A. H.; Mehta, D. R. (1959). "A Bronchodilator Alkaloid (Vasicinone) from Adhatoda vasica Nees". Nature 184 (4695): 1317. doi:10.1038/1841317a0. ISSN 0028-0836. PMID 13793186. Bibcode: 1959Natur.184.1317A.
- ↑ Mehta, D. R.; Naravane, J. S.; Desai, R. M. (1963). "Vasicinone. A Bronchodilator Principle from Adhatoda Vasica Nees (N. O. Acanthaceae)". The Journal of Organic Chemistry 28 (2): 445–448. doi:10.1021/jo01037a041. ISSN 0022-3263.
- ↑ 3.0 3.1 3.2 Rajani, M; Soni, S; Anandjiwala, Sheetal; Patel, G (2008). "Validation of different methods of preparation of Adhatoda vasica leaf juice by quantification of total alkaloids and vasicine". Indian Journal of Pharmaceutical Sciences 70 (1): 36–42. doi:10.4103/0250-474X.40329. ISSN 0250-474X. PMID 20390078.
- ↑ "Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids". Pharmacogn Rev 7 (14): 199–212. July 2013. doi:10.4103/0973-7847.120524. PMID 24347928.
- ↑ 5.0 5.1 5.2 Avula, B. (2008). "Quantitative determination of vasicine and vasicinone in Adhatoda vasica by high performance capillary electrophoresis". Die Pharmazie 63 (1): 20–22. doi:10.1691/ph.2008.7175. PMID 18271297. http://docserver.ingentaconnect.com/deliver/connect/govi/00317144/v63n1/s4.pdf?expires=1388798104&id=76777466&titleid=75007325.
 |

