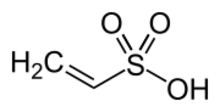
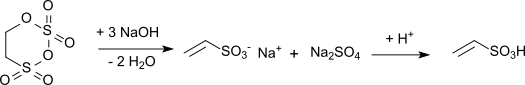Chemistry:Vinylsulfonic acid
| |
| Names | |
|---|---|
| Preferred IUPAC name
Ethenesulfonic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
| |
| |
| Properties | |
| C2H4O3S | |
| Molar mass | 108.11 g·mol−1 |
| Appearance | colouless liquid |
| Boiling point | 128 °C (1,29 hPa)[1]
* 95 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Vinylsulfonic acid is the organosulfur compound with the chemical formula CH2=CHSO3H. It is the simplest unsaturated sulfonic acid.[2][3] The C=C double bond is a site of high reactivity. Polymerization gives polyvinylsulfonic acid, especially when used as a comonomer with functionalized vinyl[4] and (meth)acrylic acid compounds.[5] It is a colorless, water-soluble liquid,[2] although commercial samples can appear yellow or even red.
Preparation
Vinylsulfonic acid is produced industrially by the alkaline hydrolysis of carbyl sulfate with subsequent acidification of the resulting vinyl sulfonate salt:[6]
The reaction is highly exothermic (reaction enthalpy: 1,675 kJ/kg) and requires exact maintenance of temperature and pH during the hydrolysis. When calcium hydroxide is used as the hydrolysis medium, a solution of calcium vinyl sulfonate is obtained. Acidification of this hydrolysis mixture with sulfuric acid gives vinylsulfonic acid, together with the poorly soluble calcium sulfate.
Vinylsulfonic acid also can be prepared by dehydration of isethionic acid with phosphorus pentoxide:[7]
Vinylsulfonic acid can also be prepared by sulfochlorination of chloroethane, dehydrohalogenation to vinylsulfonyl chloride and subsequent hydrolysis of the acid chloride.
- ClCH
2CH
3 + SO
2 + Cl
2 → ClCH
2CH
2SO
2Cl + HCl
- ClCH
- ClCH
2CH
2SO
2Cl → H
2C=CHSO
2Cl + HCl
- ClCH
- CH
2=CHSO
2Cl + H
2O → H
2C=CHSO
3H + HCl
- CH
Use
The activated C=C double bond of vinylsulfonic acid reacts readily with nucleophiles in an addition reaction. 2-Aminoethanesulfonic acid is formed with ammonia and 2-methylaminoethanesulfonic acid with methylamine.[8]
Vinylsulfonic acid is the monomer in the preparation of highly acidic or anionic homopolymers and copolymers. These polymers are used in the electronic industry as photoresists, as ion-conductive polymer electrolyte membranes (PEM) for fuel cells. For example, transparent membranes with high ion exchange capacity and proton conductivity can be produced from polyvinylsulfonic acid.[9]
Research
Vinylsulfonic acid may also be grafted to polymeric supports (e.g. polystyrene) to give highly acidic ion exchangers, which used as catalysts for esterification and Friedel-Crafts acylations.[10] Where the sulfonic acid functionality is not essential, the much more usable alkaline aqueous solution of sodium vinylsulfonate is used, which is obtained directly in the alkaline hydrolysis of the carbyl sulfate and is commercially supplied as an aqueous solution..
References
- ↑ H.C. Haas, M.S. Simon: Reactivity ratios of some monomer pairs in J. Polymer Sci. 9 (1952) 309–314, doi:10.1002/pol.1952.120090403.
- ↑ 2.0 2.1 Kosswig, Kurt (2000). "Sulfonic Acids, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry (Wiley-VCH). doi:10.1002/14356007.a25_503. ISBN 3-527-30673-0.
- ↑ H. Distler: Zur Chemie der Vinylsulfonsäure in Angew. Chem. 77 (1965) 291–311, doi:10.1002/ange.19650770704 (PDF).
- ↑ "Polymers of vinylsulfonic acid" EP patent 0643081, issued 1995-03-15, assigned to Hoechst AG
- ↑ "VINYL SULFONIC ACID, POLYMER THEREOF, AND PRODUCTION METHOD THEREOF" US patent 2011017954, issued 2011-01-27
- ↑ "Manufacture of vinyl sulfonates and vinylsulfonic acid from carbylsulfate" US patent 3872165, issued 1975-03-18, assigned to BASF
- ↑ "Preparation of ethylenesulfonic acid" US patent 2597696, issued 1952-05-20, assigned to American Cyanamid
- ↑ H. Distler (1965-04-07). "Zur Chemie der Vinylsulfonsäure". Angewandte Chemie 77 (7): 291–302. doi:10.1002/ange.19650770704. Bibcode: 1965AngCh..77..291D.
- ↑ Teruyuki Okayasu, Toshiyasu Hibino, Hiroyuki Nishide (17 May 2011), "Free Radical Polymerization Kinetics of Vinylsulfonic Acid and Highly Acidic Properties of its Polymer", Macromolecular Chemistry and Physics 212 (10): 1072–1079, doi:10.1002/macp.201000773, http://libgen.io/scimag/get.php?doi=10.1002/macp.201000773
- ↑ T. Okayasu, K. Saito, H. Nishide, M.T W. Hearn: Poly(vinylsulfonic acid)-grafted solid catalysts: new materials for acid-catalysed organic synthetic reactions. In: Green Chem. 12 (2010) 1981–1989, doi:10.1039/C0GC00241K.
 |