Chemistry:Vulgaxanthin
From HandWiki
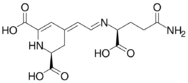
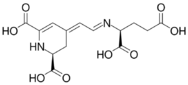
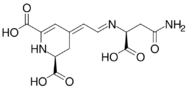
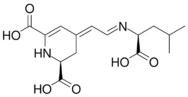
Chemical structures of vulgaxathanins
Vulgaxanthin I
Vulgaxanthin II
Vulgaxanthin III
Vulgaxanthin IV
Vulgaxanthins are a group of betaxanthins, or the predominant yellow plant pigments found in red beets, among other plants like Mirabilis jalapa and swiss chard. They are antioxidant pigments, types I, II, III, IV, and V. Like all betaxanthins, they cannot be hydrolyzed by acid to aglycone without degradation. Water activity also affects stability of this antioxidant. It has been studied as a natural nutritional additive but instability remains a problem.[1]
Reactions
- Vulgaxanthin-II + Ammonia + NADH = Vulgaxanthin-I + NAD+ + H2O
- Vulgaxanthin-I + H2O = Vulgaxanthin-II + Ammonia
- Vulgaxanthin-II + ATP + Ammonia = Vulgaxanthin-I + ADP + Orthophosphate
- Vulgaxanthin-II + ATP + Ammonia = Vulgaxanthin-I + Diphosphate + 5'-AMP
- Betalamic acid + L-Glutamine + ATP = Vulgaxanthin-I + ADP + Orthophosphate
See also
References
- ↑ Trejo-Tapia, G.; Balcazar-Aguilar, J. B.; Martínez-Bonfil, B.; Salcedo-Morales, G.; Jaramillo-Flores, M.; Arenas-Ocampo, M. L.; Jiménez-Aparicio, A. (2008-01-01). "Effect of screening and subculture on the production of betaxanthins in Beta vulgaris L. var. 'Dark Detroit' callus culture" (in en). Innovative Food Science & Emerging Technologies 9 (1): 32–36. doi:10.1016/j.ifset.2007.04.009. ISSN 1466-8564. https://www.sciencedirect.com/science/article/pii/S1466856407000641.
 |