Chemistry:Chlorophyll b
| |
| Names | |
|---|---|
| IUPAC name
Chlorophyll b
| |
| Systematic IUPAC name
Magnesium [methyl (3S,4S,21R)-14-ethyl-13-formyl-4,8,18-trimethyl-20-oxo-3-(3-oxo-3-{[(2E,7R,11R)-3,7,11,15-tetramethyl-2-hexadecen-1-yl]oxy}propyl)-9-vinyl-21-phorbinecarboxylatato(2-)-κ2N,N′] | |
| Other names
β-Chlorophyll
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C55H70MgN4O6 | |
| Molar mass | 907.492 g·mol−1 |
| Appearance | Green |
| Odor | Odorless |
| Melting point | ~ 125 °C (257 °F; 398 K)[1] |
| Insoluble[1] | |
| Solubility | Very soluble in ethanol, ether, pyridine Soluble in methanol[1] |
| Absorbance | See text |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
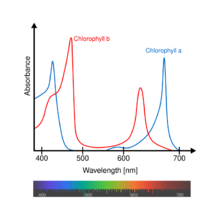
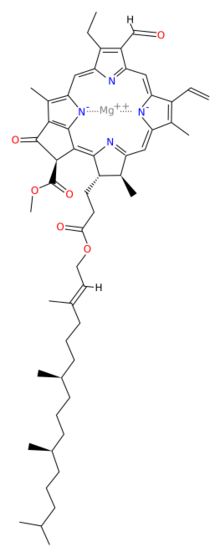
Chlorophyll b is a form of chlorophyll. Chlorophyll b helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll a in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light.[2]
In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll b. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll b to chlorophyll a.[3] This is adaptive, as increasing chlorophyll b increases the range of wavelengths absorbed by the shade chloroplasts.
 |
|
| Structure of chlorophyll b molecule showing the long hydrocarbon tail | |
Biosynthesis
The Chlorophyll b biosynthetic pathway utilizes a variety of enzymes.[4] In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme.[5][6][7] The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ALA are then reduced to porphobilinogen (PBG), and four molecules of PBG are coupled, forming protoporphyrin IX.
Chlorophyll synthase[8] is the enzyme that completes the biosynthesis of chlorophyll b[9][10] by catalysing the reaction EC 2.5.1.62
- chlorophyllide b + phytyl diphosphate chlorophyll b + diphosphate
This forms an ester of the carboxylic acid group in chlorophyllide b with the 20-carbon diterpene alcohol phytol.
References
- ↑ 1.0 1.1 1.2 Lide, David R., ed (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- ↑ "Photosynthesis pigments". http://www2.mcdaniel.edu/Biology/botf99/photo/p3igments.html.
- ↑ Kitajima, Kaoru; Hogan, Kevin P (2003). "Increases of chlorophyll a/b ratios during acclimation of tropical woody seedlings to nitrogen limitation and high light". Plant, Cell & Environment 26 (6): 857–865. doi:10.1046/j.1365-3040.2003.01017.x. PMID 12803613.
- ↑ "Genetic analysis of chlorophyll biosynthesis". Annual Review of Genetics 31 (1): 61–89. 1997. doi:10.1146/annurev.genet.31.1.61. PMID 9442890.
- ↑ Battersby, A. R. (2000). "Tetrapyrroles: the Pigments of Life. A Millennium review". Nat. Prod. Rep. 17 (6): 507–526. doi:10.1039/B002635M. PMID 11152419.
- ↑ Akhtar, M. (2007). "The Modification of Acetate and Propionate Side Chains During the Biosynthesis of Haem and Chlorophylls: Mechanistic and Stereochemical Studies". Ciba Foundation Symposium 180 - the Biosynthesis of the Tetrapyrrole Pigments. Novartis Foundation Symposia. 180. pp. 131–155. doi:10.1002/9780470514535.ch8. ISBN 9780470514535.
- ↑ Willows, Robert D. (2003). "Biosynthesis of chlorophylls from protoporphyrin IX". Natural Product Reports 20 (6): 327–341. doi:10.1039/B110549N. PMID 12828371.
- ↑ Schmid, H. C.; Rassadina, V.; Oster, U.; Schoch, S.; Rüdiger, W. (2002). "Pre-Loading of Chlorophyll Synthase with Tetraprenyl Diphosphate is an Obligatory Step in Chlorophyll Biosynthesis". Biological Chemistry 383 (11): 1769–78. doi:10.1515/BC.2002.198. PMID 12530542. https://epub.ub.uni-muenchen.de/17847/1/bc.2002.198.pdf.
- ↑ Eckhardt, Ulrich; Grimm, Bernhard; Hortensteiner, Stefan (2004). "Recent advances in chlorophyll biosynthesis and breakdown in higher plants". Plant Molecular Biology 56 (1): 1–14. doi:10.1007/s11103-004-2331-3. PMID 15604725. https://boris.unibe.ch/102079/.
- ↑ Bollivar, David W. (2007). "Recent advances in chlorophyll biosynthesis". Photosynthesis Research 90 (2): 173–194. doi:10.1007/s11120-006-9076-6. PMID 17370354.
 |
