Chemistry:Flavan
From HandWiki
| |
| Names | |
|---|---|
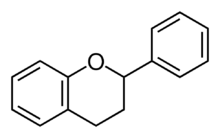
| IUPAC name
Flavan
| |
| Systematic IUPAC name
2-Phenyl-3,4-dihydro-2H-1-benzopyran | |
| Identifiers | |
3D model (JSmol)
|
|
| 383899 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C15H14O | |
| Molar mass | 210.27 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The flavans are benzopyran derivatives that use the 2-phenyl-3,4-dihydro-2H-chromene skeleton. They may be found in plants. These compounds include the flavan-3-ols, flavan-4-ols and flavan-3,4-diols (leucoanthocyanidin).
A C-glycosidic flavan can be isolated from cocoa liquor.[1]
Casuarina glauca is an actinorhizal plant producing root nitrogen-fixing nodules infested by Frankia. There is a regular pattern of cell layers containing flavans.[2]
References
- ↑ Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects. Tsutomu Hatano, Haruka Miyatake, Midori Natsume, Naomi Osakabe, Toshio Takizawa, Hideyuki Ito and Takashi Yoshida, Phytochemistry, April 2002, Volume 59, Issue 7, Pages 749–758, doi:10.1016/S0031-9422(02)00051-1
- ↑ Laplaze, L.; Gherbi, H.; Frutz, T.; Pawlowski, K.; Franche, C.; Macheix, J. J.; Auguy, F.; Bogusz, D. et al. (2002). "Flavan-Containing Cells Delimit Frankia Infected Compartments in Casuarina glauca Nodules". Nitrogen Fixation: From Molecules to Crop Productivity. Current Plant Science and Biotechnology in Agriculture. 38. pp. 455. doi:10.1007/0-306-47615-0_254. ISBN 0-7923-6233-0.
 |
