Chemistry:Zirconium stearate
From HandWiki
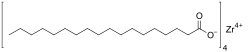
| |
| Names | |
|---|---|
| Other names
zirconium(4+) octadecanoate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C72H140ZrO8 | |
| Molar mass | 1225.1 |
| Appearance | white powder |
| Density | g/cm3 |
| Boiling point | 359.4 °C (678.9 °F; 632.5 K) |
| insoluble | |
| Hazards | |
| Flash point | 162.4 °C (324.3 °F; 435.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Zirconium stearate is a metal-organic compound, a salt of zirconium and stearic acid with the chemical formula C72H140ZrO8.[1][2]
The compound is classified as a metallic soap, i.e. a metal derivative of a fatty acid.[3][4]
Synthesis
Zirconium stearate is prepared by boiling stearic acid and sodium carbonate in water and then adding zirconium oxychloride solution.[5]
Also, zirconium stearate can be prepared by reacting zirconium nitrate and sodium oleate.[6]
Physical properties
The compound forms white powder.[7]
Uses
Zirconium stearate is used as a raw material for waterproofing materials and emulsion stabilizers.[8]
Also used as a flattening agent.[9]
References
- ↑ "zirconium stearate [15844-92-5, Information for zirconium stearate [15844-92-5], Suppliers of zirconium stearate [15844-92-5]"]. chemnet.com. http://www.chemnet.com/Global/Products/15844-92-5%20zirconiumstearate/Suppliers-1-0.html.
- ↑ "zirconium stearate - 15844-92-5 | Vulcanchem". vulcanchem.com. https://www.vulcanchem.com/product/main-products/15844-92-5.
- ↑ (in en) Occupational Exposures in Santa Clara County: Santa Clara Valley Integrated Environmental Management Project. U.S. Environmental Protection Agency, Region IX. 1986. p. 18. https://books.google.com/books?id=b5y7qkt7HQEC&dq=zirconium+stearate&pg=RA2-PA114. Retrieved 28 February 2023.
- ↑ Schick, M. J. (19 September 2017) (in en). Surface Characteristics of Fibers and Textiles: Part Ii. Routledge. p. 518. ISBN 978-1-351-41264-3. https://books.google.com/books?id=nHatDwAAQBAJ&dq=zirconium+stearate&pg=PA518. Retrieved 28 February 2023.
- ↑ (in en) The Chemical Trade Journal and Chemical Engineer. Davis Bros. (C.T.J.) Limited. 1954. p. 1060. https://books.google.com/books?id=pMzb3g7iZ-oC&q=Zirconium+stearate. Retrieved 1 March 2023.
- ↑ Mathews, Joseph Howard; Holmes, Harry Nicholls; Weiser, Harry Boyer (1926) (in en). Colloid Symposium Monograph. Williams & Wilkins Company. p. 52. https://books.google.com/books?id=mV9LAAAAMAAJ&q=Zirconium+stearate. Retrieved 1 March 2023.
- ↑ "zirconium stearate, 15844-92-5". thegoodscentscompany.com. http://www.thegoodscentscompany.com/data/rw1123301.html.
- ↑ "Zirconium Compounds | Products" (in en). Daiichi Kigenso Kagaku-Kogyo Co.. https://www.dkkk.co.jp/english/products/pro04.html.
- ↑ Kinzie, Charles J.; Eugene, Wainer (19 November 1940). "Zirconium salts of water-insoluble fatty acids and methods of making same". https://patents.google.com/patent/US2221975A/en.
 |

