Medicine:Cancer pharmacogenomics
Cancer pharmacogenomics is the study of how variances in the genome influences an individual’s response to different cancer drug treatments. It is a subset of the broader field of pharmacogenomics, which is the area of study aimed at understanding how genetic variants influence drug efficacy and toxicity.[1]
Cancer is a genetic disease where changes to genes can cause cells to grow and divide out of control. Each cancer can have a unique combination of genetic mutations, and even cells within the same tumour may have different genetic changes. In clinical settings, it has commonly been observed that the same types and doses of treatment can result in substantial differences in efficacy and toxicity across patients.[2][3] Thus, the application of pharmacogenomics within the field of cancer can offer key advantages for personalizing cancer therapy, minimizing treatment toxicity, and maximizing treatment efficacy. This can include choosing drugs that target specific mutations within cancer cells, identifying patients at risk for severe toxicity to a drug, and identifying treatments that a patient is most likely to benefit from.[4] Applying pharmacogenomics within cancer has considerable differences compared to other complex diseases, as there are two genomes that need to be considered - the germline and the tumour. The germline genome considers inter-individual inherited genetic variations, and the tumour genome considers any somatic mutations that accrue as a cancer evolves.[5] The accumulation of somatic mutations within the tumour genome represents variation in disease, and plays a major role in understanding how individuals will respond to treatments. Additionally, the germline genome affects toxicity reactions to a specific treatment due to its influence on drug exposure. Specifically, pharmacokinetic genes participate in the inactivation and elimination of active compounds.[6] Therefore, differences within the germline genome should also be considered.[5][7][8]
Strategies
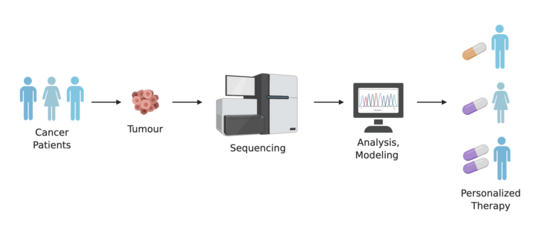
Advances in cancer diagnostics and treatment have shifted the use of traditional methods of physical examination, in vivo, and histopathological analysis to assessment of cancer drivers, mutations, and targetable genomic biomarkers.[9] There are an increasing number of genomic variants being studied and identified as potential therapeutically actionable targets and drug metabolism modifiers.[10][11] Thus, a patient's genomic information, in addition to information about the patient's tumour, can be used to determine a personalized approach to cancer treatment.[9][12]
Cancer-driven DNA alterations
Cancer-driven DNA alterations can include somatic DNA mutations and inherited DNA variants. They are not a direct focus of pharmacogenomic studies, but they can have an impact on pharmacogenomic strategies.[9] These alterations can affect the pharmacokinetics and pharmacodynamics of metabolic pathways, making them potentially actionable drug-targets.
As whole-genome technologies continue to advance, there will be increased opportunities to discover mutations and variants that are involved in tumour progression, response to therapy, and drug-metabolism.
Polymorphism search
Candidate polymorphism search refers to finding polymorphic DNA sequences within specific genes that are candidates for certain traits. Within pharmacogenomics, this method tries to resolve pharmacokinetic or pharmacodynamic traits of a compound to a candidate polymorphism level.[9][13] This type of information can contribute to selecting effective therapeutic strategies for a patient.
To understand the potential functional impact of a polymorphic DNA sequence, gene silencing can be used. Previously, siRNAs have been commonly used to suppress gene expressions, but more recently, siRNA have been suggested for use in studying and developing therapeutics.[14][15]
Another new method being applied is Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR). CRISPR, combined with the Cas9 enzyme, form the basis for the technology known as CRISPR-Cas9. This system can recognize and cleave specific DNA sequences, and thus is a powerful system for gene silencing purposes.[16]
Pathway search
An extension on the previous strategies is candidate pathway search. This type of analysis considers a group of related genes, whose altered function may have an effect on therapy, rather than solely focusing on one gene. It can provide insight into additional information such as gene-gene interactions, epistatic effects, or influences from cis-regulatory elements.[9][17] These all contribute to understanding variations in drug efficacy and toxicity between patients.
Whole-Genome Strategies
Advancements in the cost and throughput of sequencing technologies is making it possible to perform whole-genome sequencing at higher rates. The ability to perform whole-genome analysis for cancer patients can aid in identifying markers of predisposition to drug toxicity and efficacy.[18] Strategies for pharmacogenomic discovery using whole-genome sequences include targeting frequently mutated gene stretches (known as hotspots) to identify markers of prognostic and diagnostic significance, or targeting specific genes that are known to be associated with a particular disease.[9]
Gene target examples
HER2
HER2 is an established therapeutic target within breast cancer, and the activation of HER2 is observed in approximately 20% of breast cancers as a result of overexpression.[19][20] Trastuzumab, the first HER2-targeted drug developed in 1990, interferes with HER2 signalling. In 2001, a study showed that adding trastuzumab to chemotherapy improved overall survival in women with HER2-positive metastatic breast cancer.[21] Then, in 2005, it was shown that trastuzumab is effective as an adjuvant treatment in women with early-stage breast cancer.[19][22] Thus, trastuzumab has been a standard-of-care treatment in both metastatic and early stage HER2-positive breast cancer cases. Many genome sequencing studies have also revealed that other cancer tumours had HER2 alterations, including overexpression, amplifications and other mutations.[23][24][25][26] Because of this, there has been a lot of interest in studying the efficacy of HER2-targeted therapies within a range of cancer types, including bladder, colorectal, and gastro-esophageal.
BRC-ABL
The majority of chronic myelogenous leukemia cases are caused by a rearrangement between chromosomes 9 and 22. This results in the fusion of the genes BCR and ABL. This atypical gene fusion encodes for unregulated tyrosine kinase activity, which results in the rapid and continuous division of white blood cells.[20][27] Drugs known as tyrosine kinase inhibitors target BCR-ABL, and are the standard treatment for chronic myelogenous leukemia. Imatinib was the first tyrosine kinase inhibitor discovered with high specificity for targeting BCR-ABL.[28] However, after imatinib was used as the first-line therapy, several BCR-ABL-dependent and BCR-ABL-independent mechanisms of resistance developed. Thus, new second-line and third-line drugs have also been developed to address new, mutated forms of BCR-ABL. These include dasatinib, nilotinib, bosutinib, and ponatinib.[27]
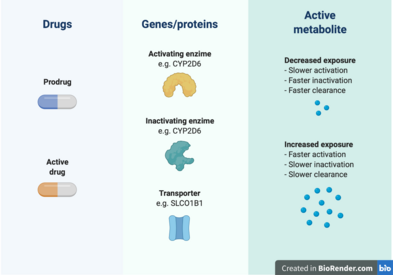
Pharmacokinetic genes
Cancer pharmacogenomics has also contributed to the understanding of how pharmacokinetic genes affect the exposure to cancer drugs, which can help predict patient sensitivity to treatment toxicity.[6] Some of these findings have been successfully translated into clinical practice in the form of professional guidelines from the Clinical Pharmacogenomics Implementation Consortium (CPIC) or other institutions.[29]
TPMT
The TPMT gene encodes for the thiopurine S-methyltransferase (TPMT) enzyme. It participates in the S-methylation of thiopurine drugs, which include 6-mercaptopurine, 6-thioguanine, and Azathioprine.[30] The first two drugs are indicated for leukemias and lymphomas, while Azathioprine is used in nonmalignant conditions such as Crohn’s disease. These purine antimetabolites are activated in the form of thioguanine nucleotides that affect DNA replication when incorporated into DNA.[6] This activation occurs through hypoxanthine phosphoribosyltransferase to 6-thioguanosines (6-TGN), and the resulting antimetabolites are inactivated by TPMT.[29] It has been established that the TPMT genotype of a patient can affect the levels of exposure to the active metabolites, which has an impact in the treatment toxicity and efficacy.[31][32] Specifically, TPMT-deficient patients, such as those homozygous for the *2 and *3 alleles, can experience myelosuppression up to pancytopenia.[33][29] In a study on 1214 European Caucasian individuals, a trimodal distribution of TPMT genotypes was found, with 89.5% normal-to-high methylators, 9.9% intermediates, and 0.6% deficient methylators[33] CPIC guidelines recommend a dose reduction of 5-10% of the standard dose and a lower frequency of application in individuals that are TPMT poor metabolizers.[34]
DPD
The dihydropyrimidine dehydrogenase (DPD) protein is responsible for the inactivation of more than 80% of the anticancer drug 5-Fluorouracil (5-FU) in the liver. This drug is commonly used in colorectal cancer treatment, and increased exposure to it can cause myelosuppression, mucositis, neurotoxicity, hand-foot syndrome, and diarrhea.[29] The genotype of DPYD (the gene that codes for DPD) has been linked to severe 5-FU toxicities in several studies summarized in meta-analyses.[35][36][37] The CPIC has provided guidelines for implementation of DPYD pharmacogenetics, indicating that homozygote carriers of low-activity variants should be prescribed an alternative drug, while heterozygotes should receive half of the normal dose.[38]
UGT1A1
The UDP glucuronosyltransferase 1A1 (UGT1A1) is an hepatic enzyme involved in the glucoronidation of exogenous and endogenous substrates, such as bilirubin.[6][39] There have been over 100 variants identified in UGT1A1 and some mutations are implicated Gilbert syndrome and Cringler-Najjar syndrome. Two variants in particular, UGT1A1*28 and UGT1A1*6, are associated with the pharmacogenomics of irinotecan chemotherapy. A UGT1A1*28 allele means the presence of 7 TA repeats in the promoter sequence of the gene, instead of the normal 6 repeats.[6] The allele UGT1A1*6 is characterized by a SNP in exon 1.[40]
Irinotecan is a prodrug[6] used in the treatment of many solid tumours, including colorectal, pancreatic, and lung cancer.[41] Irinotecan is metabolized into its active compound SN-38, which inhibits the enzyme topoisomerase-1, involved in DNA replication.[42] This active metabolite is inactivated after glucoronidation, mainly performed by UGT1A1.[39] High exposure to SN-38 can result in neutropenia and gastrointestinal toxicity.[6] The decreased activity of UGT1A1 in UGT1A1*28 individuals has been found to increase exposure to the active compound and toxicity.[43][44] For UGT1A1*6, this relationship is more controversial, with some studies finding it can predict irinotecan toxicity while others don’t.[40] Previous prospective studies for assessing the adequate dose of irinotecan in Asians have supported the usage of lower doses in patients with both of UGT1A1*28 and UGT1A1*6.[45][46] The results from these and other pharmacogenomics studies have been translated into clinical guidelines from organizations in USA, Canada, France, The Netherlands, and Europe.[41] All of these institutions recommend a dose reduction in UGT1A1*28 patients.
Challenges
One of the biggest challenges in using pharmacogenomics to study cancer is the difficulty in conducting studies in humans. Drugs used for chemotherapy are too toxic to give to healthy individuals, which makes it difficult to perform genetic studies between related individuals.[5] Furthermore, some mutations occur at high frequencies, whereas others occur at very low frequencies, so there is often a need to screen a large number of patients in order to identify those with a particular genetic marker. And, although genomic-driven analyses is effective for stratifying patients and identifying possible treatment options, it is often difficult for laboratories to get reimbursed for these genomic sequencing tests. Thus, tracking clinical outcomes for patients whom undergo sequencing is key to demonstrating both the clinical utility and cost-effectiveness of pharmacogenomics within cancer.[47]
Another challenge is that cancer patients are often treated with different combinations and dosages of drugs, so finding a large sample of patients that have been treated the same way is rare. So, studying the pharmacogenomics of a specific drug of interest is difficult, and, because additional identical trials may not be feasible, it can be difficult to replicate discoveries.[1]
Furthermore, studies have shown that drug efficacy and toxicity are likely multigenic traits. Since pathways contain multiple genes, various combinations of driver mutations could promote tumour progression.[47][48][49] This can make it difficult to distinguish between functional driver mutations versus random, nonfunctional mutations.[50]
Future
With new tools and technologies continuing to develop, there are growing opportunities to analyze cancer at the single-cell level. Corresponding approaches with whole-genome sequencing can also be applied to single-cell sequences and analyses. This level of pharmacogenomics has implications in personalized medicine, as single-cell RNA sequencing and genotyping can characterize subclones of the same tumour,[9] and lead to the identification therapy-resistant cells, as well as their corresponding pathways.[51]
As the ability to analyze and profile cancers continues to improve, so will the therapies developed to treat them. And, with increasing attention being given to whole-genome sequencing and single-cell sequencing, there will be a growing amount of pharmacogenomic data to analyze. These analyses will rely on new and improved bioinformatics tools to help identify targetable genes and pathways, to help select safer and more effect therapies for cancer patients.
References
- ↑ 1.0 1.1 "Cancer pharmacogenomics: strategies and challenges". Nature Reviews. Genetics 14 (1): 23–34. January 2013. doi:10.1038/nrg3352. PMID 23183705.
- ↑ "Pharmacogenomics: translating functional genomics into rational therapeutics". Science 286 (5439): 487–91. October 1999. doi:10.1126/science.286.5439.487. PMID 10521338.
- ↑ "No pain relief from codeine...? An introduction to pharmacogenomics". Acta Anaesthesiologica Scandinavica 45 (2): 140–9. February 2001. PMID 11167158.
- ↑ "What Is Cancer?" (in en). 2007-09-17. https://www.cancer.gov/about-cancer/understanding/what-is-cancer.
- ↑ 5.0 5.1 5.2 "Pharmacogenomics of chemotherapeutic susceptibility and toxicity". Genome Medicine 4 (11): 90. 2012. doi:10.1186/gm391. PMID 23199206.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 "Pharmacogenetics of cancer drugs". Annual Review of Medicine 66 (1): 65–81. 2015-01-14. doi:10.1146/annurev-med-053013-053944. PMID 25386932.
- ↑ "Heritability and linkage analysis of sensitivity to cisplatin-induced cytotoxicity". Cancer Research 64 (12): 4353–6. June 2004. doi:10.1158/0008-5472.CAN-04-0340. PMID 15205351.
- ↑ "Chemotherapeutic-induced apoptosis: a phenotype for pharmacogenomics studies". Pharmacogenetics and Genomics 21 (8): 476–88. August 2011. doi:10.1097/FPC.0b013e3283481967. PMID 21642893.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Concetta Crisafulli, Concetta; Romeo, Petronilla Daniela; Calabrò, Marco; Epasto, Ludovica Martina; Alberti, Saverio (2019). "Pharmacogenetic and pharmacogenomic discovery strategies" (in en-us). Cancer Drug Resistance 2 (2): 225–241. doi:10.20517/cdr.2018.008. PMID 35582724.
- ↑ "Challenges in pharmacogenetics". European Journal of Clinical Pharmacology 69 (Suppl 1): 17–23. May 2013. doi:10.1007/s00228-013-1492-x. PMID 23640184.
- ↑ "The current state of molecular testing in the treatment of patients with solid tumors, 2019". CA: A Cancer Journal for Clinicians 69 (4): 305–343. July 2019. doi:10.3322/caac.21560. PMID 31116423.
- ↑ "Next-Generation Sequencing to Diagnose Suspected Genetic Disorders". The New England Journal of Medicine 379 (14): 1353–1362. October 2018. doi:10.1056/NEJMra1711801. PMID 30281996.
- ↑ "Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome". Proceedings of the National Academy of Sciences of the United States of America 107 (50): 21611–6. December 2010. doi:10.1073/pnas.1010179107. PMID 21115826. Bibcode: 2010PNAS..10721611C.
- ↑ "Short hairpin RNA-mediated gene silencing". SiRNA Design. Methods in Molecular Biology. 942. 2013. pp. 205–32. doi:10.1007/978-1-62703-119-6_12. ISBN 978-1-62703-118-9.
- ↑ "Short hairpin RNA (shRNA): design, delivery, and assessment of gene knockdown". RNA Therapeutics. Methods in Molecular Biology. 629. 2010. pp. 141–58. doi:10.1007/978-1-60761-657-3_10. ISBN 978-1-60761-656-6.
- ↑ "CRISPR/Cas9 for genome editing: progress, implications and challenges". Human Molecular Genetics 23 (R1): R40-6. September 2014. doi:10.1093/hmg/ddu125. PMID 24651067.
- ↑ "Coupled Single-Cell CRISPR Screening and Epigenomic Profiling Reveals Causal Gene Regulatory Networks". Cell 176 (1–2): 361–376.e17. January 2019. doi:10.1016/j.cell.2018.11.022. PMID 30580963.
- ↑ "Next generation sequencing: implications in personalized medicine and pharmacogenomics". Molecular BioSystems 12 (6): 1818–30. May 2016. doi:10.1039/C6MB00115G. PMID 27066891.
- ↑ 19.0 19.1 "HER2-targeted therapies - a role beyond breast cancer". Nature Reviews. Clinical Oncology 17 (1): 33–48. January 2020. doi:10.1038/s41571-019-0268-3. PMID 31548601.
- ↑ 20.0 20.1 "Pharmacogenomics and cancer" (in en). https://www.yourgenome.org/stories/pharmacogenomics-and-cancer.
- ↑ "Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2". The New England Journal of Medicine 344 (11): 783–92. March 2001. doi:10.1056/NEJM200103153441101. PMID 11248153.
- ↑ "Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer". The New England Journal of Medicine 353 (16): 1659–72. October 2005. doi:10.1056/NEJMoa052306. PMID 16236737.
- ↑ "Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial". Lancet 376 (9742): 687–97. August 2010. doi:10.1016/S0140-6736(10)61121-X. PMID 20728210.
- ↑ "A significant subgroup of resectable gallbladder cancer patients has an HER2 positive status". Virchows Archiv 468 (4): 431–9. April 2016. doi:10.1007/s00428-015-1898-1. PMID 26758058.
- ↑ "HER2 status in colorectal cancer: its clinical significance and the relationship between HER2 gene amplification and expression". PLOS ONE 9 (5): e98528. 2014. doi:10.1371/journal.pone.0098528. PMID 24879338. Bibcode: 2014PLoSO...998528S.
- ↑ "HER2 expression status in diverse cancers: review of results from 37,992 patients". Cancer and Metastasis Reviews 34 (1): 157–64. March 2015. doi:10.1007/s10555-015-9552-6. PMID 25712293.
- ↑ 27.0 27.1 "Past, present, and future of Bcr-Abl inhibitors: from chemical development to clinical efficacy". Journal of Hematology & Oncology 11 (1): 84. June 2018. doi:10.1186/s13045-018-0624-2. PMID 29925402.
- ↑ "The interplay of structural information and functional studies in kinase drug design: insights from BCR-Abl". Current Opinion in Cell Biology 21 (2): 288–95. April 2009. doi:10.1016/j.ceb.2009.01.014. PMID 19217274.
- ↑ 29.0 29.1 29.2 29.3 "Advances and challenges in hereditary cancer pharmacogenetics". Expert Opinion on Drug Metabolism & Toxicology 13 (1): 73–82. January 2017. doi:10.1080/17425255.2017.1233965. PMID 27603572.
- ↑ "Methylation pharmacogenetics: thiopurine methyltransferase as a model system". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 22 (9–10): 1055–71. January 1992. doi:10.3109/00498259209051860. PMID 1441597.
- ↑ "Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia". Lancet 336 (8709): 225–9. July 1990. doi:10.1016/0140-6736(90)91745-V. PMID 1973780.
- ↑ "Thiopurine methyltransferase genotype predicts therapy-limiting severe toxicity from azathioprine". Annals of Internal Medicine 129 (9): 716–8. November 1998. doi:10.7326/0003-4819-129-9-199811010-00007. PMID 9841604.
- ↑ 33.0 33.1 "Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-Caucasians and identification of novel TPMT variants". Pharmacogenetics 14 (7): 407–17. July 2004. doi:10.1097/01.fpc.0000114745.08559.db. PMID 15226673.
- ↑ "Clinical Pharmacogenetics Implementation Consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing". Clinical Pharmacology and Therapeutics 89 (3): 387–91. March 2011. doi:10.1038/clpt.2010.320. PMID 21270794.
- ↑ "Genetic markers of toxicity from capecitabine and other fluorouracil-based regimens: investigation in the QUASAR2 study, systematic review, and meta-analysis". Journal of Clinical Oncology 32 (10): 1031–9. April 2014. doi:10.1200/JCO.2013.51.1857. PMID 24590654.
- ↑ "DPYD IVS14+1G>A and 2846A>T genotyping for the prediction of severe fluoropyrimidine-related toxicity: a meta-analysis". Pharmacogenomics 14 (11): 1255–72. August 2013. doi:10.2217/pgs.13.116. PMID 23930673.
- ↑ "Clinical relevance of DPYD variants c.1679T>G, c.1236G>A/HapB3, and c.1601G>A as predictors of severe fluoropyrimidine-associated toxicity: a systematic review and meta-analysis of individual patient data". The Lancet. Oncology 16 (16): 1639–50. December 2015. doi:10.1016/S1470-2045(15)00286-7. PMID 26603945.
- ↑ "Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing". Clinical Pharmacology and Therapeutics 94 (6): 640–5. December 2013. doi:10.1038/clpt.2013.172. PMID 23988873.
- ↑ 39.0 39.1 "UGT1A1 polymorphisms in cancer: impact on irinotecan treatment" (in English). Pharmacogenomics and Personalized Medicine 10: 61–68. 2017-02-28. doi:10.2147/pgpm.s108656. PMID 28280378.
- ↑ 40.0 40.1 "UGT1A1*6 polymorphisms are correlated with irinotecan-induced neutropenia: a systematic review and meta-analysis". Cancer Chemotherapy and Pharmacology 80 (1): 135–149. July 2017. doi:10.1007/s00280-017-3344-3. PMID 28585035.
- ↑ 41.0 41.1 "Individualization of Irinotecan Treatment: A Review of Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics". Clinical Pharmacokinetics 57 (10): 1229–1254. October 2018. doi:10.1007/s40262-018-0644-7. PMID 29520731.
- ↑ "Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes". The EMBO Journal 18 (5): 1397–406. March 1999. doi:10.1093/emboj/18.5.1397. PMID 10064605.
- ↑ "The role of UGT1A1*28 polymorphism in the pharmacodynamics and pharmacokinetics of irinotecan in patients with metastatic colorectal cancer". Journal of Clinical Oncology 24 (19): 3061–8. July 2006. doi:10.1200/JCO.2005.05.5400. PMID 16809730.
- ↑ "UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer". British Journal of Cancer 91 (4): 678–82. August 2004. doi:10.1038/sj.bjc.6602042. PMID 15280927.
- ↑ "Phase I study of irinotecan and doxifluridine for metastatic colorectal cancer focusing on the UGT1A1*28 polymorphism". Cancer Science 101 (3): 722–7. March 2010. doi:10.1111/j.1349-7006.2009.01428.x. PMID 20028383.
- ↑ "IN THIS ISSUE" (in en). Japanese Journal of Clinical Oncology 41 (4): NP. 2011-04-01. doi:10.1093/jjco/hyr047. ISSN 0368-2811.
- ↑ 47.0 47.1 "Cancer pharmacogenomics, challenges in implementation, and patient-focused perspectives" (in English). Pharmacogenomics and Personalized Medicine 9: 65–77. 2016-07-12. doi:10.2147/pgpm.s62918. PMID 27471406.
- ↑ "The patterns and dynamics of genomic instability in metastatic pancreatic cancer". Nature 467 (7319): 1109–13. October 2010. doi:10.1038/nature09460. PMID 20981101. Bibcode: 2010Natur.467.1109C.
- ↑ "Intratumor heterogeneity and branched evolution revealed by multiregion sequencing". The New England Journal of Medicine 366 (10): 883–892. March 2012. doi:10.1056/NEJMoa1113205. PMID 22397650.
- ↑ "The cancer genome". Nature 458 (7239): 719–24. April 2009. doi:10.1038/nature07943. PMID 19360079. Bibcode: 2009Natur.458..719S.
- ↑ "Mapping normal and cancer cell signalling networks: towards single-cell proteomics". Nature Reviews. Cancer 6 (2): 146–55. February 2006. doi:10.1038/nrc1804. PMID 16491074.
 |