Biology:CRISPR
| Cascade (CRISPR-associated complex for antiviral defense) | |||||||
|---|---|---|---|---|---|---|---|
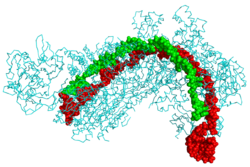 CRISPR Cascade protein (cyan) bound to CRISPR RNA (green) and phage DNA (red)[1] | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | CRISPR | ||||||
| Entrez | 947229 | ||||||
| PDB | 4QYZ (ECOD) | ||||||
| RefSeq (Prot) | NP_417241.1 | ||||||
| UniProt | P38036 | ||||||
| |||||||
| Part of a series on |
| Genetic engineering |
|---|
 |
| Genetically modified organisms |
| History and regulation |
| Process |
|
| Applications |
| Controversies |
CRISPR (/ˈkrɪspər/) (an acronym for clustered regularly interspaced short palindromic repeats) is a family of DNA sequences found in the genomes of prokaryotic organisms such as bacteria and archaea.[2] These sequences are derived from DNA fragments of bacteriophages that had previously infected the prokaryote. They are used to detect and destroy DNA from similar bacteriophages during subsequent infections. Hence these sequences play a key role in the antiviral (i.e. anti-phage) defense system of prokaryotes and provide a form of acquired immunity.[2][3][4][5] CRISPR is found in approximately 50% of sequenced bacterial genomes and nearly 90% of sequenced archaea.[6]

Cas9 (or "CRISPR-associated protein 9") is an enzyme that uses CRISPR sequences as a guide to recognize and open up specific strands of DNA that are complementary to the CRISPR sequence. Cas9 enzymes together with CRISPR sequences form the basis of a technology known as CRISPR-Cas9 that can be used to edit genes within the organisms.[8][9] This editing process has a wide variety of applications including basic biological research, development of biotechnological products, and treatment of diseases.[10][11] The development of the CRISPR-Cas9 genome editing technique was recognized by the Nobel Prize in Chemistry in 2020 which was awarded to Emmanuelle Charpentier and Jennifer Doudna.[12][13]
History
Repeated sequences
The discovery of clustered DNA repeats took place independently in three parts of the world. The first description of what would later be called CRISPR is from Osaka University researcher Yoshizumi Ishino and his colleagues in 1987. They accidentally cloned part of a CRISPR sequence together with the "iap" gene (isozyme conversion of alkaline phosphatase) from the genome of Escherichia coli[14][15] which was their target. The organization of the repeats was unusual. Repeated sequences are typically arranged consecutively, without interspersing different sequences.[11][15] They did not know the function of the interrupted clustered repeats.
In 1993, researchers of Mycobacterium tuberculosis in the Netherlands published two articles about a cluster of interrupted direct repeats (DR) in that bacterium. They recognized the diversity of the sequences that intervened in the direct repeats among different strains of M. tuberculosis[16] and used this property to design a typing method that was named spoligotyping, which is still in use, today.[17][18]
Francisco Mojica at the University of Alicante in Spain studied repeats observed in the archaeal organisms of Haloferax and Haloarcula species and their function. Mojica's supervisor surmised at the time that the clustered repeats had a role in correctly segregating replicated DNA into daughter cells during cell division because plasmids and chromosomes with identical repeat arrays could not coexist in Haloferax volcanii. Transcription of the interrupted repeats was also noted for the first time; this was the first full characterization of CRISPR.[18][19] By 2000, Mojica performed a survey of scientific literature and one of his students performed a search in published genomes with a program devised by himself. They identified interrupted repeats in 20 species of microbes as belonging to the same family.[20] Because those sequences were interspaced, Mojica initially called these sequences "short regularly spaced repeats" (SRSR).[21] In 2001, Mojica and Ruud Jansen, who were searching for an additional interrupted repeats, proposed the acronym CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) to alleviate the confusion stemming from the numerous acronyms used to describe the sequences in the scientific literature.[19][22] In 2002, Tang, et al. showed evidence that CRISPR repeat regions from the genome of Archaeoglobus fulgidus were transcribed into the long RNA molecules that were subsequently processed into unit-length small RNAs, plus some longer forms of 2, 3, or more spacer-repeat units.[23][24]
In 2005, yogurt researcher Rodolphe Barrangou discovered that Streptococcus thermophilus, after iterative phage challenges, develops increased phage resistance, and this enhanced resistance is due to the incorporation of additional CRISPR spacer sequences.[25] The Danish food company Danisco, which at that time Barrangou worked for, then developed phage-resistant S. thermophilus strains for use in yogurt production. Danisco was later bought out by DuPont, which "owns about 50 percent of the global dairy culture market" and the technology went mainstream.[26]
CRISPR-associated systems
A major addition to the understanding of CRISPR came with Jansen's observation that the prokaryote repeat cluster was accompanied by a set of homologous genes that make up CRISPR-associated systems or cas genes. Four cas genes (cas 1–4) were initially recognized. The Cas proteins showed helicase and nuclease motifs, suggesting a role in the dynamic structure of the CRISPR loci.[27] In this publication, the acronym CRISPR was used as the universal name of this pattern. However, the CRISPR function remained enigmatic.

In 2005, three independent research groups showed that some CRISPR spacers are derived from phage DNA and extrachromosomal DNA such as plasmids.[31][32][33] In effect, the spacers are fragments of DNA gathered from viruses that previously tried to attack the cell. The source of the spacers was a sign that the CRISPR-cas system could have a role in adaptive immunity in bacteria.[28][34] All three studies proposing this idea were initially rejected by high-profile journals, but eventually appeared in other journals.[35]
The first publication[32] proposing a role of CRISPR-Cas in microbial immunity, by Mojica and collaborators at the University of Alicante, predicted a role for the RNA transcript of spacers on target recognition in a mechanism that could be analogous to the RNA interference system used by eukaryotic cells. Koonin and colleagues extended this RNA interference hypothesis by proposing mechanisms of action for the different CRISPR-Cas subtypes according to the predicted function of their proteins.[36]
Experimental work by several groups revealed the basic mechanisms of CRISPR-Cas immunity. In 2007, the first experimental evidence that CRISPR was an adaptive immune system was published.[4][11] A CRISPR region in Streptococcus thermophilus acquired spacers from the DNA of an infecting bacteriophage. The researchers manipulated the resistance of S. thermophilus to different types of phages by adding and deleting spacers whose sequence matched those found in the tested phages.[37][38] In 2008, Brouns and Van der Oost identified a complex of Cas proteins (called Cascade) that in E. coli cut the CRISPR RNA precursor within the repeats into mature spacer-containing RNA molecules called CRISPR RNA (crRNA), which remained bound to the protein complex.[39] Moreover, it was found that Cascade, crRNA and a helicase/nuclease (Cas3) were required to provide a bacterial host with immunity against infection by a DNA virus. By designing an anti-virus CRISPR, they demonstrated that two orientations of the crRNA (sense/antisense) provided immunity, indicating that the crRNA guides were targeting dsDNA. That year Marraffini and Sontheimer confirmed that a CRISPR sequence of S. epidermidis targeted DNA and not RNA to prevent conjugation. This finding was at odds with the proposed RNA-interference-like mechanism of CRISPR-Cas immunity, although a CRISPR-Cas system that targets foreign RNA was later found in Pyrococcus furiosus.[11][38] A 2010 study showed that CRISPR-Cas cuts both strands of phage and plasmid DNA in S. thermophilus.[40]
Cas9
A simpler CRISPR system from Streptococcus pyogenes relies on the protein Cas9. The Cas9 endonuclease is a four-component system that includes two small molecules: crRNA and trans-activating CRISPR RNA (tracrRNA).[41][42] In 2012, Jennifer Doudna and Emmanuelle Charpentier re-engineered the Cas9 endonuclease into a more manageable two-component system by fusing the two RNA molecules into a "single-guide RNA" that, when combined with Cas9, could find and cut the DNA target specified by the guide RNA.[43] This contribution was so significant that it was recognized by the Nobel Prize in Chemistry in 2020. By manipulating the nucleotide sequence of the guide RNA, the artificial Cas9 system could be programmed to target any DNA sequence for separation.[43] Another group of collaborators comprising Virginijus Šikšnys together with Gasiūnas, Barrangou, and Horvath showed that Cas9 from the S. thermophilus CRISPR system can also be reprogrammed to target a site of their choosing by changing the sequence of its crRNA. These advances fueled efforts to edit genomes with the modified CRISPR-Cas9 system.[18]
Groups led by Feng Zhang and George Church simultaneously published descriptions of genome editing in human cell cultures using CRISPR-Cas9 for the first time.[11][44][45] It has since been used in a wide range of organisms, including baker's yeast (Saccharomyces cerevisiae),[46][47][48] the opportunistic pathogen Candida albicans,[49][50] zebrafish (Danio rerio),[51] fruit flies (Drosophila melanogaster),[52][53] ants (Harpegnathos saltator[54] and Ooceraea biroi[55]), mosquitoes (Aedes aegypti[56]), nematodes (Caenorhabditis elegans),[57] plants,[58] mice (Mus musculus domesticus),[59][60] monkeys[61] and human embryos.[62]
CRISPR has been modified to make programmable transcription factors that allows targeting and activation or silencing specific genes.[63]
The CRISPR-Cas9 system has shown to make effective gene edits in Human tripronuclear zygotes first described in a 2015 paper by Chinese scientists P. Liang and Y. Xu. The system made a successful cleavage of mutant Beta-Hemoglobin (HBB) in 28 out of 54 embryos. Four out of the 28 embryos were successfully recombined using a donor template given by the scientists. The scientists showed that during DNA recombination of the cleaved strand, the homologous endogenous sequence HBD competes with the exogenous donor template. DNA repair in human embryos is much more complicated and particular than in derived stem cells.[64]
Cas12a
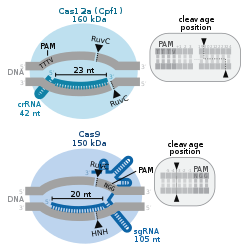
In 2015, the nuclease Cas12a (formerly known as Cpf1[65]) was characterized in the CRISPR-Cpf1 system of the bacterium Francisella novicida.[66][67] Its original name, from a TIGRFAMs protein family definition built in 2012, reflects the prevalence of its CRISPR-Cas subtype in the Prevotella and Francisella lineages. Cas12a showed several key differences from Cas9 including: causing a 'staggered' cut in double stranded DNA as opposed to the 'blunt' cut produced by Cas9, relying on a 'T rich' PAM (providing alternative targeting sites to Cas9), and requiring only a CRISPR RNA (crRNA) for successful targeting. By contrast, Cas9 requires both crRNA and a trans-activating crRNA (tracrRNA).
These differences may give Cas12a some advantages over Cas9. For example, Cas12a's small crRNAs are ideal for multiplexed genome editing, as more of them can be packaged in one vector than can Cas9's sgRNAs. The sticky 5′ overhangs left by Cas12a can also be used for DNA assembly that is much more target-specific than traditional restriction enzyme cloning.[68] Finally, Cas12a cleaves DNA 18–23 base pairs downstream from the PAM site. This means there is no disruption to the recognition sequence after repair, and so Cas12a enables multiple rounds of DNA cleavage. By contrast, since Cas9 cuts only 3 base pairs upstream of the PAM site, the NHEJ pathway results in indel mutations that destroy the recognition sequence, thereby preventing further rounds of cutting. In theory, repeated rounds of DNA cleavage should cause an increased opportunity for the desired genomic editing to occur.[69] A distinctive feature of Cas12a, as compared to Cas9, is that after cutting its target, Cas12a remains bound to the target and then cleaves other ssDNA molecules non-discriminately.[70] This property is called "collateral cleavage" or "trans-cleavage" activity and has been exploited for the development of various diagnostic technologies.[71][72]
Cas13
In 2016, the nuclease Cas13a (formerly known as C2c2) from the bacterium Leptotrichia shahii was characterized. Cas13 is an RNA-guided RNA endonuclease, which means that it does not cleave DNA, but only single-stranded RNA. Cas13 is guided by its crRNA to a ssRNA target and binds and cleaves the target. Similar to Cas12a, the Cas13 remains bound to the target and then cleaves other ssRNA molecules non-discriminately.[73] This collateral cleavage property has been exploited for the development of various diagnostic technologies.[74][75][76]
In 2021, Dr. Hui Yang characterized novel miniature Cas13 protein (mCas13) variants, Cas13X and Cas13Y. Using a small portion of N gene sequence from SARS-CoV-2 as a target in characterization of mCas13, revealed the sensitivity and specificity of mCas13 coupled with RT-LAMP for detection of SARS-CoV-2 in both synthetic and clinical samples over other available standard tests like RT-qPCR (1 copy/μL).[77]
Locus structure
Repeats and spacers
The CRISPR array is made up of an AT-rich leader sequence followed by short repeats that are separated by unique spacers.[78] CRISPR repeats typically range in size from 28 to 37 base pairs (bps), though there can be as few as 23 bp and as many as 55 bp.[79] Some show dyad symmetry, implying the formation of a secondary structure such as a stem-loop ('hairpin') in the RNA, while others are designed to be unstructured. The size of spacers in different CRISPR arrays is typically 32 to 38 bp (range 21 to 72 bp).[79] New spacers can appear rapidly as part of the immune response to phage infection.[80] There are usually fewer than 50 units of the repeat-spacer sequence in a CRISPR array.[79]
CRISPR RNA structures
Cas genes and CRISPR subtypes
Small clusters of cas genes are often located next to CRISPR repeat-spacer arrays. Collectively the 93 cas genes are grouped into 35 families based on sequence similarity of the encoded proteins. 11 of the 35 families form the cas core, which includes the protein families Cas1 through Cas9. A complete CRISPR-Cas locus has at least one gene belonging to the cas core.[81]
CRISPR-Cas systems fall into two classes. Class 1 systems use a complex of multiple Cas proteins to degrade foreign nucleic acids. Class 2 systems use a single large Cas protein for the same purpose. Class 1 is divided into types I, III, and IV; class 2 is divided into types II, V, and VI.[82] The 6 system types are divided into 19 subtypes.[83] Each type and most subtypes are characterized by a "signature gene" found almost exclusively in the category. Classification is also based on the complement of cas genes that are present. Most CRISPR-Cas systems have a Cas1 protein. The phylogeny of Cas1 proteins generally agrees with the classification system,[84] but exceptions exist due to module shuffling.[81] Many organisms contain multiple CRISPR-Cas systems suggesting that they are compatible and may share components.[85][86] The sporadic distribution of the CRISPR-Cas subtypes suggests that the CRISPR-Cas system is subject to horizontal gene transfer during microbial evolution.
This table is missing information about UniProt and InterPro cross-reference. (October 2020) |
| Class | Cas type | Cas subtype | Signature protein | Function | Reference |
|---|---|---|---|---|---|
| 1 | I | ||||
| I-A | Cas8a, Cas5 | Cas8 is a Subunit of the interference module that is important in targeting of invading DNA by recognizing the PAM sequence. Cas5 is required for processing and stability of crRNAs. | [84][87] | ||
| I-B | Cas8b | ||||
| I-C | Cas8c | ||||
| I-E | Cse1, Cse2 | ||||
| I-F | Csy1, Csy2, Csy3 | Type IF-3 have been implicated in CRISPR-associated transposons | [84] | ||
| I-G[Note 1] | GSU0054 | [88] | |||
| III | — | Cas10 | Homolog of Cas10d and Cse1. Binds CRISPR target RNA and promotes stability of the interference complex | [89][90] | |
| III-A | Csm2 | Not determined | [84] | ||
| III-B | Cmr5 | Not determined | [84] | ||
| III-C | Cas10 or Csx11 | [84][90] | |||
| III-D | Csx10 | [84] | |||
| III-E | [88] | ||||
| III-F | [88] | ||||
| IV | — | Csf1 | [88] | ||
| IV-A | [88] | ||||
| IV-B | [88] | ||||
| IV-C | [88] | ||||
| 2 | II | ||||
| II-A | Csn2 | Ring-shaped DNA-binding protein. Involved in primed adaptation in Type II CRISPR system. | [91] | ||
| II-B | Cas4 | Endonuclease that works with cas1 and cas2 to generate spacer sequences | [92] | ||
| II-C | Characterized by the absence of either Csn2 or Cas4 | [93] | |||
| V | — | Cas12 | Nuclease RuvC. Lacks HNH. | [82][94] | |
| V-A | Cas12a (Cpf1) | Auto-processing pre-crRNA activity for multiplex gene regulation | [88][95] | ||
| V-B | Cas12b (C2c1) | [88] | |||
| V-C | Cas12c (C2c3) | [88] | |||
| V-D | Cas12d (CasY) | [88] | |||
| V-E | Cas12e (CasX) | [88] | |||
| V-F | Cas12f (Cas14, C2c10) | [88] | |||
| V-G | Cas12g | [88] | |||
| V-H | Cas12h | [88] | |||
| V-I | Cas12i | [88] | |||
| V-K[Note 2] | Cas12k (C2c5) | Type V-K have been implicated in CRISPR-associated transposons. | [88] | ||
| V-U | C2c4, C2c8, C2c9 | [88] | |||
| VI | |||||
| VI-A | Cas13a (C2c2) | [88] | |||
| VI-B | Cas13b | [88] | |||
| VI-C | Cas13c | [88] | |||
| VI-D | Cas13d | [88] | |||
| VI-X | Cas13x.1 | RNA dependent RNA polymerase, Prophylactic RNA-virus inhibition | [96] | ||
| VI-Y | [96] |
Mechanism
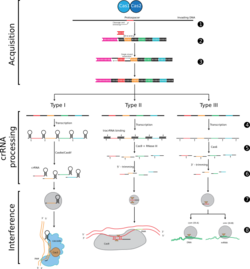
(1) Acquisition begins by recognition of invading DNA by Cas1 and Cas2 and cleavage of a protospacer.
(2) The protospacer is ligated to the direct repeat adjacent to the leader sequence and
(3) single strand extension repairs the CRISPR and duplicates the direct repeat. The crRNA processing and interference stages occur differently in each of the three major CRISPR systems.
(4) The primary CRISPR transcript is cleaved by cas genes to produce crRNAs.
(5) In type I systems Cas6e/Cas6f cleave at the junction of ssRNA and dsRNA formed by hairpin loops in the direct repeat. Type II systems use a trans-activating (tracr) RNA to form dsRNA, which is cleaved by Cas9 and RNaseIII. Type III systems use a Cas6 homolog that does not require hairpin loops in the direct repeat for cleavage.
(6) In type II and type III systems secondary trimming is performed at either the 5' or 3' end to produce mature crRNAs.
(7) Mature crRNAs associate with Cas proteins to form interference complexes.
(8) In type I and type II systems, interactions between the protein and PAM sequence are required for degradation of invading DNA. Type III systems do not require a PAM for successful degradation and in type III-A systems basepairing occurs between the crRNA and mRNA rather than the DNA, targeted by type III-B systems.

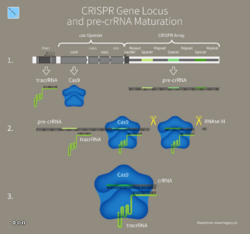
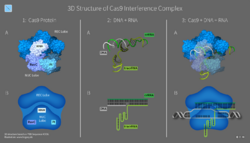

CRISPR-Cas immunity is a natural process of bacteria and archaea.[97] CRISPR-Cas prevents bacteriophage infection, conjugation and natural transformation by degrading foreign nucleic acids that enter the cell.[38]
Spacer acquisition
When a microbe is invaded by a bacteriophage, the first stage of the immune response is to capture phage DNA and insert it into a CRISPR locus in the form of a spacer. Cas1 and Cas2 are found in both types of CRISPR-Cas immune systems, which indicates that they are involved in spacer acquisition. Mutation studies confirmed this hypothesis, showing that removal of Cas1 or Cas2 stopped spacer acquisition, without affecting CRISPR immune response.[98][99][100][101][102]
Multiple Cas1 proteins have been characterised and their structures resolved.[103][104][105] Cas1 proteins have diverse amino acid sequences. However, their crystal structures are similar and all purified Cas1 proteins are metal-dependent nucleases/integrases that bind to DNA in a sequence-independent manner.[85] Representative Cas2 proteins have been characterised and possess either (single strand) ssRNA-[106] or (double strand) dsDNA-[107][108] specific endoribonuclease activity.
In the I-E system of E. coli Cas1 and Cas2 form a complex where a Cas2 dimer bridges two Cas1 dimers.[109] In this complex Cas2 performs a non-enzymatic scaffolding role,[109] binding double-stranded fragments of invading DNA, while Cas1 binds the single-stranded flanks of the DNA and catalyses their integration into CRISPR arrays.[110][111][112] New spacers are usually added at the beginning of the CRISPR next to the leader sequence creating a chronological record of viral infections.[113] In E. coli a histone like protein called integration host factor (IHF), which binds to the leader sequence, is responsible for the accuracy of this integration.[114] IHF also enhances integration efficiency in the type I-F system of Pectobacterium atrosepticum.[115] but in other systems, different host factors may be required[116]
Protospacer adjacent motifs (PAM)
Bioinformatic analysis of regions of phage genomes that were excised as spacers (termed protospacers) revealed that they were not randomly selected but instead were found adjacent to short (3–5 bp) DNA sequences termed protospacer adjacent motifs (PAM). Analysis of CRISPR-Cas systems showed PAMs to be important for type I and type II, but not type III systems during acquisition.[33][117][118][119][120][121] In type I and type II systems, protospacers are excised at positions adjacent to a PAM sequence, with the other end of the spacer cut using a ruler mechanism, thus maintaining the regularity of the spacer size in the CRISPR array.[122][123] The conservation of the PAM sequence differs between CRISPR-Cas systems and appears to be evolutionarily linked to Cas1 and the leader sequence.[121][124]
New spacers are added to a CRISPR array in a directional manner,[31] occurring preferentially,[80][117][118][125][126] but not exclusively, adjacent[120][123] to the leader sequence. Analysis of the type I-E system from E. coli demonstrated that the first direct repeat adjacent to the leader sequence is copied, with the newly acquired spacer inserted between the first and second direct repeats.[101][122]
The PAM sequence appears to be important during spacer insertion in type I-E systems. That sequence contains a strongly conserved final nucleotide (nt) adjacent to the first nt of the protospacer. This nt becomes the final base in the first direct repeat.[102][127][128] This suggests that the spacer acquisition machinery generates single-stranded overhangs in the second-to-last position of the direct repeat and in the PAM during spacer insertion. However, not all CRISPR-Cas systems appear to share this mechanism as PAMs in other organisms do not show the same level of conservation in the final position.[124] It is likely that in those systems, a blunt end is generated at the very end of the direct repeat and the protospacer during acquisition.
Insertion variants
Analysis of Sulfolobus solfataricus CRISPRs revealed further complexities to the canonical model of spacer insertion, as one of its six CRISPR loci inserted new spacers randomly throughout its CRISPR array, as opposed to inserting closest to the leader sequence.[123]
Multiple CRISPRs contain many spacers to the same phage. The mechanism that causes this phenomenon was discovered in the type I-E system of E. coli. A significant enhancement in spacer acquisition was detected where spacers already target the phage, even mismatches to the protospacer. This 'priming' requires the Cas proteins involved in both acquisition and interference to interact with each other. Newly acquired spacers that result from the priming mechanism are always found on the same strand as the priming spacer.[102][127][128] This observation led to the hypothesis that the acquisition machinery slides along the foreign DNA after priming to find a new protospacer.[128]
Biogenesis
CRISPR-RNA (crRNA), which later guides the Cas nuclease to the target during the interference step, must be generated from the CRISPR sequence. The crRNA is initially transcribed as part of a single long transcript encompassing much of the CRISPR array.[29] This transcript is then cleaved by Cas proteins to form crRNAs. The mechanism to produce crRNAs differs among CRISPR-Cas systems. In type I-E and type I-F systems, the proteins Cas6e and Cas6f respectively, recognise stem-loops[129][130][131] created by the pairing of identical repeats that flank the crRNA.[132] These Cas proteins cleave the longer transcript at the edge of the paired region, leaving a single crRNA along with a small remnant of the paired repeat region.
Type III systems also use Cas6, however, their repeats do not produce stem-loops. Cleavage instead occurs by the longer transcript wrapping around the Cas6 to allow cleavage just upstream of the repeat sequence.[133][134][135]
Type II systems lack the Cas6 gene and instead utilize RNaseIII for cleavage. Functional type II systems encode an extra small RNA that is complementary to the repeat sequence, known as a trans-activating crRNA (tracrRNA).[41] Transcription of the tracrRNA and the primary CRISPR transcript results in base pairing and the formation of dsRNA at the repeat sequence, which is subsequently targeted by RNaseIII to produce crRNAs. Unlike the other two systems, the crRNA does not contain the full spacer, which is instead truncated at one end.[136]
CrRNAs associate with Cas proteins to form ribonucleotide complexes that recognize foreign nucleic acids. CrRNAs show no preference between the coding and non-coding strands, which is indicative of an RNA-guided DNA-targeting system.[5][40][98][102][137][138][139] The type I-E complex (commonly referred to as Cascade) requires five Cas proteins bound to a single crRNA.[140][141]
Interference
During the interference stage in type I systems, the PAM sequence is recognized on the crRNA-complementary strand and is required along with crRNA annealing. In type I systems correct base pairing between the crRNA and the protospacer signals a conformational change in Cascade that recruits Cas3 for DNA degradation.
Type II systems rely on a single multifunctional protein, Cas9, for the interference step.[136] Cas9 requires both the crRNA and the tracrRNA to function and cleave DNA using its dual HNH and RuvC/RNaseH-like endonuclease domains. Basepairing between the PAM and the phage genome is required in type II systems. However, the PAM is recognized on the same strand as the crRNA (the opposite strand to type I systems).
Type III systems, like type I require six or seven Cas proteins binding to crRNAs.[142][143] The type III systems analysed from S. solfataricus and P. furiosus both target the mRNA of phages rather than phage DNA genome,[86][143] which may make these systems uniquely capable of targeting RNA-based phage genomes.[85] Type III systems were also found to target DNA in addition to RNA using a different Cas protein in the complex, Cas10.[144] The DNA cleavage was shown to be transcription dependent.[145]
The mechanism for distinguishing self from foreign DNA during interference is built into the crRNAs and is therefore likely common to all three systems. Throughout the distinctive maturation process of each major type, all crRNAs contain a spacer sequence and some portion of the repeat at one or both ends. It is the partial repeat sequence that prevents the CRISPR-Cas system from targeting the chromosome as base pairing beyond the spacer sequence signals self and prevents DNA cleavage.[146] RNA-guided CRISPR enzymes are classified as type V restriction enzymes.
Evolution
| CRISPR associated protein Cas2 (adaptation RNase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
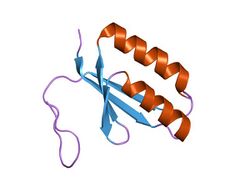 Crystal structure of a hypothetical protein tt1823 from Thermus thermophilus | |||||||||
| Identifiers | |||||||||
| Symbol | CRISPR_Cas2 | ||||||||
| Pfam | PF09827 | ||||||||
| InterPro | IPR019199 | ||||||||
| CDD | cd09638 | ||||||||
| |||||||||
| CRISPR-associated protein CasA/Cse1 (Type I effector DNase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | CRISPR_Cse1 | ||||||||
| Pfam | PF09481 | ||||||||
| InterPro | IPR013381 | ||||||||
| CDD | cd09729 | ||||||||
| |||||||||
| CRISPR associated protein CasC/Cse3/Cas6 (Type I effector RNase) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystal structure of a crispr-associated protein from Thermus thermophilus | |||||||||
| Identifiers | |||||||||
| Symbol | CRISPR_assoc | ||||||||
| Pfam | PF08798 | ||||||||
| Pfam clan | CL0362 | ||||||||
| InterPro | IPR010179 | ||||||||
| CDD | cd09727 | ||||||||
| |||||||||
The cas genes in the adaptor and effector modules of the CRISPR-Cas system are believed to have evolved from two different ancestral modules. A transposon-like element called casposon encoding the Cas1-like integrase and potentially other components of the adaptation module was inserted next to the ancestral effector module, which likely functioned as an independent innate immune system.[147] The highly conserved cas1 and cas2 genes of the adaptor module evolved from the ancestral module while a variety of class 1 effector cas genes evolved from the ancestral effector module.[148] The evolution of these various class 1 effector module cas genes was guided by various mechanisms, such as duplication events.[149] On the other hand, each type of class 2 effector module arose from subsequent independent insertions of mobile genetic elements.[150] These mobile genetic elements took the place of the multiple gene effector modules to create single gene effector modules that produce large proteins which perform all the necessary tasks of the effector module.[150] The spacer regions of CRISPR-Cas systems are taken directly from foreign mobile genetic elements and thus their long-term evolution is hard to trace.[151] The non-random evolution of these spacer regions has been found to be highly dependent on the environment and the particular foreign mobile genetic elements it contains.[152]
CRISPR-Cas can immunize bacteria against certain phages and thus halt transmission. For this reason, Koonin described CRISPR-Cas as a Lamarckian inheritance mechanism.[153] However, this was disputed by a critic who noted, "We should remember [Lamarck] for the good he contributed to science, not for things that resemble his theory only superficially. Indeed, thinking of CRISPR and other phenomena as Lamarckian only obscures the simple and elegant way evolution really works".[154] But as more recent studies have been conducted, it has become apparent that the acquired spacer regions of CRISPR-Cas systems are indeed a form of Lamarckian evolution because they are genetic mutations that are acquired and then passed on.[155] On the other hand, the evolution of the Cas gene machinery that facilitates the system evolves through classic Darwinian evolution.[155]
Coevolution
Analysis of CRISPR sequences revealed coevolution of host and viral genomes.[156] Cas9 proteins are highly enriched in pathogenic and commensal bacteria. CRISPR-Cas-mediated gene regulation may contribute to the regulation of endogenous bacterial genes, particularly during interaction with eukaryotic hosts. For example, Francisella novicida uses a unique, small, CRISPR-Cas-associated RNA (scaRNA) to repress an endogenous transcript encoding a bacterial lipoprotein that is critical for F. novicida to dampen host response and promote virulence.[157]
The basic model of CRISPR evolution is newly incorporated spacers driving phages to mutate their genomes to avoid the bacterial immune response, creating diversity in both the phage and host populations. To resist a phage infection, the sequence of the CRISPR spacer must correspond perfectly to the sequence of the target phage gene. Phages can continue to infect their hosts' given point mutations in the spacer.[146] Similar stringency is required in PAM or the bacterial strain remains phage sensitive.[118][146]
Rates
A study of 124 S. thermophilus strains showed that 26% of all spacers were unique and that different CRISPR loci showed different rates of spacer acquisition.[117] Some CRISPR loci evolve more rapidly than others, which allowed the strains' phylogenetic relationships to be determined. A comparative genomic analysis showed that E. coli and S. enterica evolve much more slowly than S. thermophilus. The latter's strains that diverged 250,000 years ago still contained the same spacer complement.[158]
Metagenomic analysis of two acid-mine-drainage biofilms showed that one of the analyzed CRISPRs contained extensive deletions and spacer additions versus the other biofilm, suggesting a higher phage activity/prevalence in one community than the other.[80] In the oral cavity, a temporal study determined that 7–22% of spacers were shared over 17 months within an individual while less than 2% were shared across individuals.[126]
From the same environment, a single strain was tracked using PCR primers specific to its CRISPR system. Broad-level results of spacer presence/absence showed significant diversity. However, this CRISPR added three spacers over 17 months,[126] suggesting that even in an environment with significant CRISPR diversity some loci evolve slowly.
CRISPRs were analysed from the metagenomes produced for the Human Microbiome Project.[159] Although most were body-site specific, some within a body site are widely shared among individuals. One of these loci originated from streptococcal species and contained ≈15,000 spacers, 50% of which were unique. Similar to the targeted studies of the oral cavity, some showed little evolution over time.[159]
CRISPR evolution was studied in chemostats using S. thermophilus to directly examine spacer acquisition rates. In one week, S. thermophilus strains acquired up to three spacers when challenged with a single phage.[160] During the same interval, the phage developed single-nucleotide polymorphisms that became fixed in the population, suggesting that targeting had prevented phage replication absent these mutations.[160]
Another S. thermophilus experiment showed that phages can infect and replicate in hosts that have only one targeting spacer. Yet another showed that sensitive hosts can exist in environments with high-phage titres.[161] The chemostat and observational studies suggest many nuances to CRISPR and phage (co)evolution.
Identification
CRISPRs are widely distributed among bacteria and archaea[162] and show some sequence similarities.[132] Their most notable characteristic is their repeating spacers and direct repeats. This characteristic makes CRISPRs easily identifiable in long sequences of DNA, since the number of repeats decreases the likelihood of a false positive match.[163]
Analysis of CRISPRs in metagenomic data is more challenging, as CRISPR loci do not typically assemble, due to their repetitive nature or through strain variation, which confuses assembly algorithms. Where many reference genomes are available, polymerase chain reaction (PCR) can be used to amplify CRISPR arrays and analyse spacer content.[117][126][164][165][166][167] However, this approach yields information only for specifically targeted CRISPRs and for organisms with sufficient representation in public databases to design reliable polymerase PCR primers. Degenerate repeat-specific primers can be used to amplify CRISPR spacers directly from environmental samples; amplicons containing two or three spacers can be then computationally assembled to reconstruct long CRISPR arrays.[167]
The alternative is to extract and reconstruct CRISPR arrays from shotgun metagenomic data. This is computationally more difficult, particularly with second generation sequencing technologies (e.g. 454, Illumina), as the short read lengths prevent more than two or three repeat units appearing in a single read. CRISPR identification in raw reads has been achieved using purely de novo identification[168] or by using direct repeat sequences in partially assembled CRISPR arrays from contigs (overlapping DNA segments that together represent a consensus region of DNA)[159] and direct repeat sequences from published genomes[169] as a hook for identifying direct repeats in individual reads.
Use by phages
Another way for bacteria to defend against phage infection is by having chromosomal islands. A subtype of chromosomal islands called phage-inducible chromosomal island (PICI) is excised from a bacterial chromosome upon phage infection and can inhibit phage replication.[170] PICIs are induced, excised, replicated, and finally packaged into small capsids by certain staphylococcal temperate phages. PICIs use several mechanisms to block phage reproduction. In the first mechanism, PICI-encoded Ppi differentially blocks phage maturation by binding or interacting specifically with phage TerS, hence blocking phage TerS/TerL complex formation responsible for phage DNA packaging. In the second mechanism PICI CpmAB redirects the phage capsid morphogenetic protein to make 95% of SaPI-sized capsid and phage DNA can package only 1/3rd of their genome in these small capsids and hence become nonviable phage.[171] The third mechanism involves two proteins, PtiA and PtiB, that target the LtrC, which is responsible for the production of virion and lysis proteins. This interference mechanism is modulated by a modulatory protein, PtiM, binds to one of the interference-mediating proteins, PtiA, and hence achieves the required level of interference.[172]
One study showed that lytic ICP1 phage, which specifically targets Vibrio cholerae serogroup O1, has acquired a CRISPR-Cas system that targets a V. cholera PICI-like element. The system has 2 CRISPR loci and 9 Cas genes. It seems to be homologous to the I-F system found in Yersinia pestis. Moreover, like the bacterial CRISPR-Cas system, ICP1 CRISPR-Cas can acquire new sequences, which allows phage and host to co-evolve.[173][174]
Certain archaeal viruses were shown to carry mini-CRISPR arrays containing one or two spacers. It has been shown that spacers within the virus-borne CRISPR arrays target other viruses and plasmids, suggesting that mini-CRISPR arrays represent a mechanism of heterotypic superinfection exclusion and participate in interviral conflicts.[167]
Applications
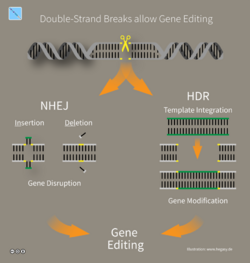
CRISPR gene editing
CRISPR technology has been applied in the food and farming industries to engineer probiotic cultures and to immunize industrial cultures (for yogurt, for instance) against infections. It is also being used in crops to enhance yield, drought tolerance and nutritional value.[175][176][177] CRISPR gene editing has also become a fantastic tool for scientific research. The amplification and "knock-out" of gene products is a reliable way to identify genes of interest for pharmaceutical development or to simple better understand the complexities that lie in any genome.
By the end of 2014, some 1,000 research papers had been published that mentioned CRISPR.[178][179] The technology had been used to functionally inactivate genes in human cell lines and cells, to study Candida albicans, to modify yeasts used to make biofuels, and genetically modify crop strains.[179] Hsu and his colleagues state that the ability to manipulate the genetic sequences allows for reverse engineering that can positively affect biofuel production.[180] CRISPR can also be used to change mosquitoes so they cannot transmit diseases such as malaria.[181] CRISPR-based approaches utilizing Cas12a have recently been utilized in the successful modification of a broad number of plant species.[182]
In July 2019, CRISPR was used to experimentally treat a patient with a genetic disorder. The patient was a 34-year-old woman with sickle cell disease.[183]
In February 2020, progress was made on HIV treatments with 60–80% of the integrated viral DNA removed in mice and some being completely free from the virus after edits involving both LASER ART, a new anti-retroviral therapy, and CRISPR.[184]
In March 2020, CRISPR-modified virus was injected into a patient's eye in an attempt to treat Leber congenital amaurosis.[185]
In the future, CRISPR gene editing could potentially be used to create new species or revive extinct species from closely related ones.[186]
CRISPR-based re-evaluations of claims for gene-disease relationships have led to the discovery of potentially important anomalies.[187][188]
In July 2021, CRISPR gene editing of hiPSC's was used to study the role of MBNL proteins associated with DM1.[189]
The CRISPR technique has a positive response in working towards different disorders like Nervous system, Circulatory system, Stem cells, blood disorders, muscular degeneration. This tool has made advanced approaches in both therapeutic and biomedical systems and some of the applications are discussed below,
1.1 β-Hemoglobinopathies
This disease comes under genetic disorders which are caused by mutation occurring in the structure of hemoglobin or due to substitution of different amino acids in globin chains. Due to this, the red blood cells (RBC) cause a string of obstacles such as failure of heart, hindrance of blood vessels, defects in growth and optical problems.[190] To rehabilitate β-hemoglobinopathies, the patient's multipotent cells are transferred in a mice model to study the rate of gene therapy in ex-vivo which results in expression of mRNA and the gene being rectified. Intriguingly RBC half-life was also increased.
1.2 Hemophilia
It is a loss of function in blood where clotting factors do not work properly. There are two types, Hemophilia A and Hemophilia B. By using CRISPR-Cas9, a vector is inserted into bacteria.[191] The vector used is Adenoviral vector which helps in correction of genes. Doubtlessly, CRISPR has given hope for the treatment of hemophilia by setting right the genes.
1.3 Neurological disorders
CRISPR is used in suppressing the mutations which cause gain of function and also repairs the mutations with loss of functions with gene editing in neurological disorders.[192] The gene editing tool setup a foothold in vivo application for assimilation of molecular pathways.
1.4 Blindness
The Eye disorders became more impediment for the doctors to treat the victims. Moreover, the retinal tissue present in the eye is free from body immune response. The most commonly occurring worldwide eye diseases are cataract and retinitis pigmentosa (RP). These are caused by a missense mutation in the alpha chain that leads to permanent blindness. The approach of CRISPR is to bag the gene coding retinal protein and edit the genome which results in good vision.
1.5 Cardiovascular diseases
The CRISPR technology works more efficiently towards diseases related to the heart. Due to deposition of cholesterol in the walls of the artery leads to blockage of flow of blood. This is caused by mutation in low density lipoprotein cholesterol receptors (LDLC) which results in release of cholesterol into blood in higher levels.[193] This can be treated by deletion of base pair in exon 4 of LDLC receptor. This is a nonsense mutation.
Applications of CRISPR in agriculture
The application of CRISPR in plants was successfully achieved in the year 2013. CRISPR Cas9 has become an influential appliance in editing genomes in crops. It made a mark in present breeding systems,[194]
2.1 Boosting the yield
For high production of yield in cereals the balance of cytokinin is changed. cytokinin oxidase/dehydrogenase (CKX), is an enzyme,[195] so the gene that codes this enzyme was knocked out for more yield to be produced.
2.2 Enhancing quality
Grains have a high amount of amylose polysaccharide. To decrease the amylose content CRISPR is used to alter the amino acids which leads to low production of saccharide. Moreover, wheat contains gluten proteins due to which some of them are intolerant to gluten and cause a disorder called coeliac disease.[196] The gene editing tool targets the gluten genes which results in low gluten production in wheat.
2.3 Resistance to disease
The biotic stress of plants can be reduced by using CRISPR tools. The bacterial infections caused on the rice leads to activation of transcription of genes,[197] the products of these are susceptible to disease and by using CRISPR scientists were able to generate lines of resistance.
General applications of CRISPR
3.1 Gene Therapy
The overall genetic disorders discovered till now are about 6000. Most of them do not have treatment till date. The role of gene therapy is to substitute with exogenous DNA in the place of defective genes and edit the mutated sequence.[198] This therapy made a huge impact in medical biotechnology.
3.2 Base editing
They are two types of base editings:
Cytidine base editor is a novel therapy in which the cytidine (C) changes to thymidine (T).
Adenine base editor (ABE),[199] in this there is a change in base complements from adenine (A) to Guanine (G).
The mutations were directly installed in cellular DNA so that the donor template is not required. The base editings can only edit point mutations moreover they can only fix up to four-point mutations.[200] So, to master this problem CRISPR system has introduced a new technique known as Cas9 fusion to stretch the level of genome editing.
3.3 Gene silencing and activating
Furthermore, the CRISPR Cas9 protein can modulate genes either by activating or silencing based on genes of interest.[201] There is a nuclease called dCas9 (endonuclease) used to silence or activate the expression of genes.
Limitations in Applications of CRISPR
The researchers are facing many challenges in gene editing.[202] The major hurdles coming in the clinical applications are ethical issues and the transport system to the target site. As the units of CRISPR system taken from bacteria, when they are transferred to host cells it produces an immune response against them. Physical, chemical, viral vectors are used as vehicles to deliver the complex into the host.[citation needed] Due to this many complications are arising such as cell damage that leads to cell death. In the case of viral vectors, the capacity of the virus is small and Cas9 protein is large. So, to overcome these new methods were developed in which smaller strains of Cas9 are taken from bacteria. Finally, a great extent of work is still needed to improve the system.
CRISPR as diagnostic tool

CRISPR associated nucleases have shown to be useful as a tool for molecular testing due to their ability to specifically target nucleic acid sequences in a high background of non-target sequences.[204] In 2016, the Cas9 nuclease was used to deplete unwanted nucleotide sequences in next-generation sequencing libraries while requiring only 250 picograms of initial RNA input.[205] Beginning in 2017, CRISPR associated nucleases were also used for direct diagnostic testing of nucleic acids, down to single molecule sensitivity.[74][206] CRISPR diversity is used as an analysis target to discern phylogeny and diversity in bacteria, such as in xanthomonads by Martins et al., 2019.[207]: 552 Early detections of plant pathogens by molecular typing of the pathogen's CRISPRs can be used in agriculture as demonstrated by Shen et al., 2020.[207]: 553
By coupling CRISPR-based diagnostics to additional enzymatic processes, the detection of molecules beyond nucleic acids is possible. One example of a coupled technology is SHERLOCK-based Profiling of IN vitro Transcription (SPRINT). SPRINT can be used to detect a variety of substances, such as metabolites in patient samples or contaminants in environmental samples, with high throughput or with portable point-of-care devices.[76] CRISPR-Cas platforms are also being explored for detection[71][72][203][208][209] and inactivation of SARS-CoV-2, the virus that causes COVID-19.[210] Two different comprehensive diagnostic tests, AIOD-CRISPR and SHERLOCK test have been identified for SARS-CoV-2.[211] The SHERLOCK test is based on a fluorescently labelled press reporter RNA which has the ability to identify 10 copies per microliter.[212] The AIOD-CRISPR helps with robust and highly sensitive visual detection of the viral nucleic acid.[213]
See also
- CRISPR activation
- Anti-CRISPR
- CRISPR/Cas Tools
- CRISPR gene editing
- The CRISPR Journal
- DRACO
- Gene knockout
- Genetics
- Genome-wide CRISPR-Cas9 knockout screens
- Glossary of genetics
- Human Nature (2019 documentary film)
- MAGESTIC
- Prime editing
- RNAi
- SiRNA
- Surveyor nuclease assay
- Synthetic biology
- Zinc finger
Notes
References
- ↑ PDB: 4QYZ: "Crystal structure of a CRISPR RNA–guided surveillance complex bound to a ssDNA target". Science 345 (6203): 1479–1484. 2014. doi:10.1126/science.1256996. PMID 25123481. Bibcode: 2014Sci...345.1479M.
- ↑ 2.0 2.1 "The roles of CRISPR-Cas systems in adaptive immunity and beyond". Current Opinion in Immunology 32: 36–41. 2015. doi:10.1016/j.coi.2014.12.008. PMID 25574773.
- ↑ "What is CRISPR/Cas9?". Archives of Disease in Childhood: Education and Practice Edition 101 (4): 213–215. August 2016. doi:10.1136/archdischild-2016-310459. PMID 27059283.
- ↑ 4.0 4.1 "CRISPR provides acquired resistance against viruses in prokaryotes". Science 315 (5819): 1709–1712. March 2007. doi:10.1126/science.1138140. PMID 17379808. Bibcode: 2007Sci...315.1709B. (registration required)
- ↑ 5.0 5.1 "CRISPR interference limits horizontal gene transfer in staphylococci by targeting DNA". Science 322 (5909): 1843–1845. December 2008. doi:10.1126/science.1165771. PMID 19095942. Bibcode: 2008Sci...322.1843M.
- ↑ "The Biology of CRISPR-Cas: Backward and Forward". Cell 172 (6): 1239–1259. March 2018. doi:10.1016/j.cell.2017.11.032. PMID 29522745.
- ↑ "CRISPR/Cas, the immune system of bacteria and archaea". Science 327 (5962): 167–170. January 2010. doi:10.1126/science.1179555. PMID 20056882. Bibcode: 2010Sci...327..167H.
- ↑ "Gene Editing on Center Stage". Trends in Genetics 34 (8): 600–611. August 2018. doi:10.1016/j.tig.2018.05.004. PMID 29908711.
- ↑ "CRISPR/Cas9 for genome editing: progress, implications and challenges". Human Molecular Genetics 23 (R1): R40–6. 2014. doi:10.1093/hmg/ddu125. PMID 24651067.
- ↑ CRISPR-CAS9, TALENS and ZFNS – the battle in gene editing https://www.ptglab.com/news/blog/crispr-cas9-talens-and-zfns-the-battle-in-gene-editing/
- ↑ 11.0 11.1 11.2 11.3 11.4 "Development and applications of CRISPR-Cas9 for genome engineering". Cell 157 (6): 1262–1278. June 2014. doi:10.1016/j.cell.2014.05.010. PMID 24906146.
- ↑ "Press release: The Nobel Prize in Chemistry 2020". Nobel Foundation. https://www.nobelprize.org/prizes/chemistry/2020/press-release/.
- ↑ "Nobel Prize in Chemistry Awarded to 2 Scientists for Work on Genome Editing – Emmanuelle Charpentier and Jennifer A. Doudna developed the Crispr tool, which can alter the DNA of animals, plants and microorganisms with high precision.". The New York Times. 7 October 2020. https://www.nytimes.com/2020/10/07/science/nobel-prize-chemistry-crispr.html.
- ↑ "Cysteine proteases: Battling pathogenic parasitic protozoans with omnipresent enzymes". Microbiological Research 249: 126784. August 2021. doi:10.1016/j.micres.2021.126784. PMID 33989978.
- ↑ 15.0 15.1 "Nucleotide sequence of the iap gene, responsible for alkaline phosphatase isozyme conversion in Escherichia coli, and identification of the gene product". Journal of Bacteriology 169 (12): 5429–5433. December 1987. doi:10.1128/jb.169.12.5429-5433.1987. PMID 3316184.
- ↑ "Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis". Journal of Clinical Microbiology 31 (8): 1987–1995. August 1993. doi:10.1128/JCM.31.8.1987-1995.1993. PMID 7690367.
- ↑ "Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method". Molecular Microbiology 10 (5): 1057–1065. December 1993. doi:10.1111/j.1365-2958.1993.tb00976.x. PMID 7934856.
- ↑ 18.0 18.1 18.2 "On the Origin of CRISPR-Cas Technology: From Prokaryotes to Mammals". Trends in Microbiology 24 (10): 811–820. 2016. doi:10.1016/j.tim.2016.06.005. PMID 27401123.
- ↑ 19.0 19.1 "The discovery of CRISPR in archaea and bacteria". The FEBS Journal 283 (17): 3162–3169. 2016. doi:10.1111/febs.13766. PMID 27234458. http://rua.ua.es/dspace/bitstream/10045/57676/2/2016_Mojica_Rodriguez_TheFEBSJournal_accepted.pdf.
- ↑ "Biological significance of a family of regularly spaced repeats in the genomes of Archaea, Bacteria and mitochondria". Molecular Microbiology 36 (1): 244–246. April 2000. doi:10.1046/j.1365-2958.2000.01838.x. PMID 10760181.
- ↑ The Code Breaker: Jennifer Doudna, Gene Editing, and the Future of the Human Race. New York: Simon & Schuster. 2021. p. 73. ISBN 978-1-9821-1585-2. OCLC 1239982737. https://books.google.com/books?id=f_D3DwAAQBAJ.
- ↑ CRISPR-Cas Systems : RNA-mediated Adaptive Immunity in Bacteria and Archaea. Heidelberg: Springer. 2013. p. 6. ISBN 978-3-642-34656-9.
- ↑ "Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus". Proceedings of the National Academy of Sciences of the United States of America 99 (11): 7536–7541. May 2002. doi:10.1073/pnas.112047299. PMID 12032318. Bibcode: 2002PNAS...99.7536T.
- ↑ "Biogenesis pathways of RNA guides in archaeal and bacterial CRISPR-Cas adaptive immunity". FEMS Microbiology Reviews 39 (3): 428–441. May 2015. doi:10.1093/femsre/fuv023. PMID 25994611.
- ↑ "Dairy lactococcal and streptococcal phage-host interactions: an industrial perspective in an evolving phage landscape". FEMS Microbiology Reviews 44 (6): 909–932. November 2020. doi:10.1093/femsre/fuaa048. PMID 33016324.
- ↑ "The WIRED Guide to Crispr". Wired Magazine. Condé Nast. 1 August 2020. https://www.wired.com/story/wired-guide-to-crispr/.
- ↑ "Identification of genes that are associated with DNA repeats in prokaryotes". Molecular Microbiology 43 (6): 1565–1575. March 2002. doi:10.1046/j.1365-2958.2002.02839.x. PMID 11952905.
- ↑ 28.0 28.1 "CRISPR/Cas, the immune system of bacteria and archaea". Science 327 (5962): 167–170. January 2010. doi:10.1126/Science.1179555. PMID 20056882. Bibcode: 2010Sci...327..167H.
- ↑ 29.0 29.1 "CRISPR interference: RNA-directed adaptive immunity in bacteria and archaea". Nature Reviews Genetics 11 (3): 181–190. March 2010. doi:10.1038/nrg2749. PMID 20125085.
- ↑ "The CRISPRdb database and tools to display CRISPRs and to generate dictionaries of spacers and repeats". BMC Bioinformatics 8: 172. May 2007. doi:10.1186/1471-2105-8-172. PMID 17521438.
- ↑ 31.0 31.1 "CRISPR elements in Yersinia pestis acquire new repeats by preferential uptake of bacteriophage DNA, and provide additional tools for evolutionary studies". Microbiology 151 (Pt 3): 653–663. March 2005. doi:10.1099/mic.0.27437-0. PMID 15758212.
- ↑ 32.0 32.1 "Intervening sequences of regularly spaced prokaryotic repeats derive from foreign genetic elements". Journal of Molecular Evolution 60 (2): 174–182. February 2005. doi:10.1007/s00239-004-0046-3. PMID 15791728. Bibcode: 2005JMolE..60..174M.
- ↑ 33.0 33.1 "Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin". Microbiology 151 (Pt 8): 2551–2561. August 2005. doi:10.1099/mic.0.28048-0. PMID 16079334.
- ↑ "What history tells us XXXVII. CRISPR-Cas: The discovery of an immune system in prokaryotes". Journal of Biosciences 40 (2): 221–223. June 2015. doi:10.1007/s12038-015-9532-6. PMID 25963251.
- ↑ "The Heroes of CRISPR". Cell 164 (1–2): 18–28. January 2016. doi:10.1016/j.cell.2015.12.041. PMID 26771483.
- ↑ "A putative RNA-interference-based immune system in prokaryotes: computational analysis of the predicted enzymatic machinery, functional analogies with eukaryotic RNAi, and hypothetical mechanisms of action". Biology Direct 1: 7. March 2006. doi:10.1186/1745-6150-1-7. PMID 16545108.
- ↑ "The CRISPR craze". Science 341 (6148): 833–836. August 2013. doi:10.1126/science.341.6148.833. PMID 23970676. Bibcode: 2013Sci...341..833P.
- ↑ 38.0 38.1 38.2 "CRISPR-Cas immunity in prokaryotes". Nature 526 (7571): 55–61. October 2015. doi:10.1038/nature15386. PMID 26432244. Bibcode: 2015Natur.526...55M.
- ↑ "Small CRISPR RNAs guide antiviral defense in prokaryotes". Science 321 (5891): 960–964. August 2008. doi:10.1126/science.1159689. PMID 18703739. Bibcode: 2008Sci...321..960B.
- ↑ 40.0 40.1 "The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA". Nature 468 (7320): 67–71. November 2010. doi:10.1038/nature09523. PMID 21048762. Bibcode: 2010Natur.468...67G.
- ↑ 41.0 41.1 "CRISPR RNA maturation by trans-encoded small RNA and host factor RNase III". Nature 471 (7340): 602–607. March 2011. doi:10.1038/nature09886. PMID 21455174. Bibcode: 2011Natur.471..602D.
- ↑ "Diversity of CRISPR-Cas immune systems and molecular machines". Genome Biology 16: 247. November 2015. doi:10.1186/s13059-015-0816-9. PMID 26549499.
- ↑ 43.0 43.1 "A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity". Science 337 (6096): 816–821. August 2012. doi:10.1126/science.1225829. PMID 22745249. Bibcode: 2012Sci...337..816J.
- ↑ "Multiplex genome engineering using CRISPR/Cas systems". Science 339 (6121): 819–823. February 2013. doi:10.1126/science.1231143. PMID 23287718. Bibcode: 2013Sci...339..819C.
- ↑ "RNA-guided human genome engineering via Cas9". Science 339 (6121): 823–826. February 2013. doi:10.1126/science.1232033. PMID 23287722. Bibcode: 2013Sci...339..823M.
- ↑ "Genome engineering in Saccharomyces cerevisiae using CRISPR-Cas systems". Nucleic Acids Research 41 (7): 4336–4343. April 2013. doi:10.1093/nar/gkt135. PMID 23460208.
- ↑ "Construction of a quadruple auxotrophic mutant of an industrial polyploid saccharomyces cerevisiae strain by using RNA-guided Cas9 nuclease". Applied and Environmental Microbiology 80 (24): 7694–7701. December 2014. doi:10.1128/AEM.02310-14. PMID 25281382. Bibcode: 2014ApEnM..80.7694Z.
- ↑ "Metabolic Engineering of Probiotic Saccharomyces boulardii". Applied and Environmental Microbiology 82 (8): 2280–2287. April 2016. doi:10.1128/AEM.00057-16. PMID 26850302. Bibcode: 2016ApEnM..82.2280L.
- ↑ "Candida albicans CRISPR system permits genetic engineering of essential genes and gene families". Science Advances 1 (3): e1500248. 2015. doi:10.1126/sciadv.1500248. PMID 25977940. Bibcode: 2015SciA....1E0248V.
- ↑ "Candida albicans by Increased Single Guide RNA Expression". mSphere 2 (2): e00385–16. 2017. doi:10.1128/mSphere.00385-16. PMID 28435892.
- ↑ "Efficient genome editing in zebrafish using a CRISPR-Cas system". Nature Biotechnology 31 (3): 227–229. March 2013. doi:10.1038/nbt.2501. PMID 23360964.
- ↑ "Genome engineering of Drosophila with the CRISPR RNA-guided Cas9 nuclease". Genetics 194 (4): 1029–1035. August 2013. doi:10.1534/genetics.113.152710. PMID 23709638.
- ↑ "Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system". Cell Reports 4 (1): 220–228. July 2013. doi:10.1016/j.celrep.2013.06.020. PMID 23827738.
- ↑ "An Engineered orco Mutation Produces Aberrant Social Behavior and Defective Neural Development in Ants". Cell 170 (4): 736–747.e9. August 2017. doi:10.1016/j.cell.2017.06.051. PMID 28802043.
- ↑ "orco Mutagenesis Causes Loss of Antennal Lobe Glomeruli and Impaired Social Behavior in Ants". Cell 170 (4): 727–735.e10. August 2017. doi:10.1016/j.cell.2017.07.001. PMID 28802042.
- ↑ "Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti". Cell Reports 11 (1): 51–60. April 2015. doi:10.1016/j.celrep.2015.03.009. PMID 25818303.
- ↑ "Heritable genome editing in C. elegans via a CRISPR-Cas9 system". Nature Methods 10 (8): 741–743. August 2013. doi:10.1038/nmeth.2532. PMID 23817069.
- ↑ "Demonstration of CRISPR/Cas9/sgRNA-mediated targeted gene modification in Arabidopsis, tobacco, sorghum and rice". Nucleic Acids Research 41 (20): e188. November 2013. doi:10.1093/nar/gkt780. PMID 23999092.
- ↑ "One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering". Cell 153 (4): 910–918. May 2013. doi:10.1016/j.cell.2013.04.025. PMID 23643243.
- ↑ "Deubiquitinase function of A20 maintains and repairs endothelial barrier after lung vascular injury". Cell Death Discovery 4 (60): 60. May 2018. doi:10.1038/s41420-018-0056-3. PMID 29796309.
- ↑ "Targeted genome editing in primate embryos". Cell Research 25 (7): 767–768. July 2015. doi:10.1038/cr.2015.64. PMID 26032266.
- ↑ "Biotechnology. A prudent path forward for genomic engineering and germline gene modification". Science 348 (6230): 36–38. April 2015. doi:10.1126/science.aab1028. PMID 25791083. Bibcode: 2015Sci...348...36B.
- ↑ "CRISPR interference (CRISPRi) for sequence-specific control of gene expression". Nature Protocols 8 (11): 2180–2196. November 2013. doi:10.1038/nprot.2013.132. PMID 24136345.
- ↑ "CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes". Protein & Cell 6 (5): 363–372. May 2015. doi:10.1007/s13238-015-0153-5. PMID 25894090.
- ↑ "CRISPR-Cas12a-Assisted Recombineering in Bacteria". Applied and Environmental Microbiology 83 (17). September 2017. doi:10.1128/AEM.00947-17. PMID 28646112. Bibcode: 2017ApEnM..83E.947Y.
- ↑ "Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system". Cell 163 (3): 759–771. October 2015. doi:10.1016/j.cell.2015.09.038. PMID 26422227.
- ↑ "The CRISPR-associated DNA-cleaving enzyme Cpf1 also processes precursor CRISPR RNA". Nature 532 (7600): 517–521. April 2016. doi:10.1038/nature17945. PMID 27096362. Bibcode: 2016Natur.532..517F.
- ↑ "CRISPR/Cpf1-mediated DNA-free plant genome editing". Nature Communications 8 (14406): 14406. February 2017. doi:10.1038/ncomms14406. PMID 28205546. Bibcode: 2017NatCo...814406K.
- ↑ "Cpf1 Nuclease". https://www.abmgood.com/marketing/knowledge_base/CRISPR_Cas9_Introduction_Part7.php#ATSPC.
- ↑ "CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity". Science 360 (6387): 436–439. April 2018. doi:10.1126/science.aar6245. PMID 29449511. Bibcode: 2018Sci...360..436C.
- ↑ 71.0 71.1 "CRISPR-Cas12-based detection of SARS-CoV-2". Nature Biotechnology 38 (7): 870–874. July 2020. doi:10.1038/s41587-020-0513-4. PMID 32300245.
- ↑ 72.0 72.1 "Enhancement of trans-cleavage activity of Cas12a with engineered crRNA enables amplified nucleic acid detection". Nature Communications 11 (1): 4906. September 2020. doi:10.1038/s41467-020-18615-1. PMID 32999292. Bibcode: 2020NatCo..11.4906N.
- ↑ "C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector". Science 353 (6299): aaf5573. August 2016. doi:10.1126/science.aaf5573. PMID 27256883.
- ↑ 74.0 74.1 "Nucleic acid detection with CRISPR-Cas13a/C2c2". Science 356 (6336): 438–442. April 2017. doi:10.1126/science.aam9321. PMID 28408723. Bibcode: 2017Sci...356..438G.
- ↑ "Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6". Science 360 (6387): 439–444. April 2018. doi:10.1126/science.aaq0179. PMID 29449508. Bibcode: 2018Sci...360..439G.
- ↑ 76.0 76.1 "SPRINT: a Cas13a-based platform for detection of small molecules". Nucleic Acids Research 48 (17): e101. September 2020. doi:10.1093/nar/gkaa673. PMID 32797156.
- ↑ "A Novel Miniature CRISPR-Cas13 System for SARS-CoV-2 Diagnostics". ACS Synthetic Biology 10 (10): 2541–2551. October 2021. doi:10.1021/acssynbio.1c00181. PMID 34546709.
- ↑ "CRISPR-Cas: biology, mechanisms and relevance". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 371 (1707): 20150496. November 2016. doi:10.1098/rstb.2015.0496. PMID 27672148.
- ↑ 79.0 79.1 79.2 "CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity". Molecular Cell 54 (2): 234–244. April 2014. doi:10.1016/j.molcel.2014.03.011. PMID 24766887.
- ↑ 80.0 80.1 80.2 "Rapidly evolving CRISPRs implicated in acquired resistance of microorganisms to viruses". Environmental Microbiology 10 (1): 200–207. January 2008. doi:10.1111/j.1462-2920.2007.01444.x. PMID 17894817. Bibcode: 2008EnvMi..10..200T.
- ↑ 81.0 81.1 "Origins and evolution of CRISPR-Cas systems". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences 374 (1772): 20180087. May 2019. doi:10.1098/rstb.2018.0087. PMID 30905284.
- ↑ 82.0 82.1 "Biology and Applications of CRISPR Systems: Harnessing Nature's Toolbox for Genome Engineering". Cell 164 (1–2): 29–44. January 2016. doi:10.1016/j.cell.2015.12.035. PMID 26771484.
- ↑ "Evolution and Ecology of CRISPR". Annual Review of Ecology, Evolution, and Systematics 47 (1): 307–331. November 2016. doi:10.1146/annurev-ecolsys-121415-032428.
- ↑ 84.0 84.1 84.2 84.3 84.4 84.5 84.6 84.7 "An updated evolutionary classification of CRISPR-Cas systems". Nature Reviews. Microbiology 13 (11): 722–736. November 2015. doi:10.1038/nrmicro3569. PMID 26411297.
- ↑ 85.0 85.1 85.2 "RNA-guided genetic silencing systems in bacteria and archaea". Nature 482 (7385): 331–338. February 2012. doi:10.1038/nature10886. PMID 22337052. Bibcode: 2012Natur.482..331W.
- ↑ 86.0 86.1 "A novel interference mechanism by a type IIIB CRISPR-Cmr module in Sulfolobus". Molecular Microbiology 87 (5): 1088–1099. March 2013. doi:10.1111/mmi.12152. PMID 23320564.
- ↑ "A complex of Cas proteins 5, 6, and 7 is required for the biogenesis and stability of clustered regularly interspaced short palindromic repeats (crispr)-derived rnas (crrnas) in Haloferax volcanii". The Journal of Biological Chemistry 289 (10): 7164–77. March 2014. doi:10.1074/jbc.M113.508184. PMID 24459147.
- ↑ 88.00 88.01 88.02 88.03 88.04 88.05 88.06 88.07 88.08 88.09 88.10 88.11 88.12 88.13 88.14 88.15 88.16 88.17 88.18 88.19 88.20 88.21 88.22 "Evolutionary classification of CRISPR-Cas systems: a burst of class 2 and derived variants". Nature Reviews. Microbiology 18 (2): 67–83. February 2020. doi:10.1038/s41579-019-0299-x. PMID 31857715.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid21756346 - ↑ 90.0 90.1 "Genetic Dissection of the Type III-A CRISPR-Cas System Csm Complex Reveals Roles of Individual Subunits". Cell Reports 26 (10): 2753–2765.e4. March 2019. doi:10.1016/j.celrep.2019.02.029. PMID 30840895.
- ↑ "Crystal structure of clustered regularly interspaced short palindromic repeats (CRISPR)-associated Csn2 protein revealed Ca2+-dependent double-stranded DNA binding activity". The Journal of Biological Chemistry 286 (35): 30759–30768. September 2011. doi:10.1074/jbc.M111.256263. PMID 21697083.
- ↑ "The Cas4-Cas1-Cas2 complex mediates precise prespacer processing during CRISPR adaptation". eLife 8. April 2019. doi:10.7554/eLife.44248. PMID 31021314.
- ↑ "The tracrRNA and Cas9 families of type II CRISPR-Cas immunity systems". RNA Biology 10 (5): 726–737. May 2013. doi:10.4161/rna.24321. PMID 23563642.
- ↑ "SnapShot: Class 2 CRISPR-Cas Systems". Cell 168 (1–2): 328–328.e1. January 2017. doi:10.1016/j.cell.2016.12.038. PMID 28086097.
- ↑ "CRISPR-Cas12a: Functional overview and applications". Biomedical Journal 43 (1): 8–17. February 2020. doi:10.1016/j.bj.2019.10.005. PMID 32200959.
- ↑ 96.0 96.1 "Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes". Nature Methods 18 (5): 499–506. May 2021. doi:10.1038/s41592-021-01124-4. PMID 33941935.
- ↑ "CRISPR/Cas: From Tumor Gene Editing to T Cell-Based Immunotherapy of Cancer". Frontiers in Immunology 11: 2062. 2020. doi:10.3389/fimmu.2020.02062. PMID 33117331.
- ↑ 98.0 98.1 "RNA-based viral immunity initiated by the Dicer family of host immune receptors". Immunological Reviews 227 (1): 176–188. January 2009. doi:10.1111/j.1600-065X.2008.00722.x. PMID 19120484.
- ↑ "High-resolution transcriptome maps reveal strain-specific regulatory features of multiple Campylobacter jejuni isolates". PLOS Genetics 9 (5): e1003495. May 2013. doi:10.1371/journal.pgen.1003495. PMID 23696746.
- ↑ "Mature clustered, regularly interspaced, short palindromic repeats RNA (crRNA) length is measured by a ruler mechanism anchored at the precursor processing site". Proceedings of the National Academy of Sciences of the United States of America 108 (52): 21218–21222. December 2011. doi:10.1073/pnas.1112832108. PMID 22160698. Bibcode: 2011PNAS..10821218H.
- ↑ 101.0 101.1 "Proteins and DNA elements essential for the CRISPR adaptation process in Escherichia coli". Nucleic Acids Research 40 (12): 5569–5576. July 2012. doi:10.1093/nar/gks216. PMID 22402487.
- ↑ 102.0 102.1 102.2 102.3 "CRISPR interference directs strand specific spacer acquisition". PLOS ONE 7 (4): e35888. 2012. doi:10.1371/journal.pone.0035888. PMID 22558257. Bibcode: 2012PLoSO...735888S.
- ↑ "A dual function of the CRISPR-Cas system in bacterial antivirus immunity and DNA repair". Molecular Microbiology 79 (2): 484–502. January 2011. doi:10.1111/j.1365-2958.2010.07465.x. PMID 21219465.
- ↑ "SSO1450—a CAS1 protein from Sulfolobus solfataricus P2 with high affinity for RNA and DNA". FEBS Letters 583 (12): 1928–1932. June 2009. doi:10.1016/j.febslet.2009.04.047. PMID 19427858.
- ↑ "Structural basis for DNase activity of a conserved protein implicated in CRISPR-mediated genome defense". Structure 17 (6): 904–912. June 2009. doi:10.1016/j.str.2009.03.019. PMID 19523907.
- ↑ "A novel family of sequence-specific endoribonucleases associated with the clustered regularly interspaced short palindromic repeats". The Journal of Biological Chemistry 283 (29): 20361–20371. July 2008. doi:10.1074/jbc.M803225200. PMID 18482976.
- ↑ "Structure of a CRISPR-associated protein Cas2 from Desulfovibrio vulgaris". Acta Crystallographica Section F 66 (Pt 12): 1552–1556. December 2010. doi:10.1107/S1744309110039801. PMID 21139194.
- ↑ "Double-stranded endonuclease activity in Bacillus halodurans clustered regularly interspaced short palindromic repeats (CRISPR)-associated Cas2 protein". The Journal of Biological Chemistry 287 (43): 35943–35952. October 2012. doi:10.1074/jbc.M112.382598. PMID 22942283.
- ↑ 109.0 109.1 "Cas1-Cas2 complex formation mediates spacer acquisition during CRISPR-Cas adaptive immunity". Nature Structural & Molecular Biology 21 (6): 528–534. June 2014. doi:10.1038/nsmb.2820. PMID 24793649.
- ↑ "Integrase-mediated spacer acquisition during CRISPR-Cas adaptive immunity". Nature 519 (7542): 193–198. March 2015. doi:10.1038/nature14237. PMID 25707795. Bibcode: 2015Natur.519..193N.
- ↑ "Structural and Mechanistic Basis of PAM-Dependent Spacer Acquisition in CRISPR-Cas Systems". Cell 163 (4): 840–853. November 2015. doi:10.1016/j.cell.2015.10.008. PMID 26478180.
- ↑ "Foreign DNA capture during CRISPR-Cas adaptive immunity". Nature 527 (7579): 535–538. November 2015. doi:10.1038/nature15760. PMID 26503043. Bibcode: 2015Natur.527..535N.
- ↑ "CRISPR-mediated adaptive immune systems in bacteria and archaea". Annual Review of Biochemistry 82 (1): 237–266. 2013. doi:10.1146/annurev-biochem-072911-172315. PMID 23495939.
- ↑ "CRISPR Immunological Memory Requires a Host Factor for Specificity". Molecular Cell 62 (6): 824–833. June 2016. doi:10.1016/j.molcel.2016.04.027. PMID 27211867.
- ↑ "Spacer capture and integration by a type I-F Cas1-Cas2–3 CRISPR adaptation complex". Proceedings of the National Academy of Sciences of the United States of America 114 (26): E5122–E5128. June 2017. doi:10.1073/pnas.1618421114. PMID 28611213. Bibcode: 2017PNAS..114E5122F.
- ↑ "Prespacer processing and specific integration in a Type I-A CRISPR system". Nucleic Acids Research 46 (3): 1007–1020. February 2018. doi:10.1093/nar/gkx1232. PMID 29228332.
- ↑ 117.0 117.1 117.2 117.3 "Diversity, activity, and evolution of CRISPR loci in Streptococcus thermophilus". Journal of Bacteriology 190 (4): 1401–1412. February 2008. doi:10.1128/JB.01415-07. PMID 18065539.
- ↑ 118.0 118.1 118.2 "Phage response to CRISPR-encoded resistance in Streptococcus thermophilus". Journal of Bacteriology 190 (4): 1390–1400. February 2008. doi:10.1128/JB.01412-07. PMID 18065545.
- ↑ "Short motif sequences determine the targets of the prokaryotic CRISPR defence system". Microbiology 155 (Pt 3): 733–740. March 2009. doi:10.1099/mic.0.023960-0. PMID 19246744.
- ↑ 120.0 120.1 "CRISPR families of the crenarchaeal genus Sulfolobus: bidirectional transcription and dynamic properties". Molecular Microbiology 72 (1): 259–272. April 2009. doi:10.1111/j.1365-2958.2009.06641.x. PMID 19239620.
- ↑ 121.0 121.1 "Distribution of CRISPR spacer matches in viruses and plasmids of crenarchaeal acidothermophiles and implications for their inhibitory mechanism". Biochemical Society Transactions 37 (Pt 1): 23–28. February 2009. doi:10.1042/BST0370023. PMID 19143596.
- ↑ 122.0 122.1 "CRISPR-spacer integration reporter plasmids reveal distinct genuine acquisition specificities among CRISPR-Cas I-E variants of Escherichia coli". RNA Biology 10 (5): 792–802. May 2013. doi:10.4161/rna.24023. PMID 23445770.
- ↑ 123.0 123.1 123.2 "Selective and hyperactive uptake of foreign DNA by adaptive immune systems of an archaeon via two distinct mechanisms". Molecular Microbiology 85 (6): 1044–1056. September 2012. doi:10.1111/j.1365-2958.2012.08171.x. PMID 22834906.
- ↑ 124.0 124.1 "Protospacer recognition motifs: mixed identities and functional diversity". RNA Biology 10 (5): 891–899. May 2013. doi:10.4161/rna.23764. PMID 23403393.
- ↑ "Virus population dynamics and acquired virus resistance in natural microbial communities". Science 320 (5879): 1047–1050. May 2008. doi:10.1126/science.1157358. PMID 18497291. Bibcode: 2008Sci...320.1047A.
- ↑ 126.0 126.1 126.2 126.3 "Analysis of streptococcal CRISPRs from human saliva reveals substantial sequence diversity within and between subjects over time". Genome Research 21 (1): 126–136. January 2011. doi:10.1101/gr.111732.110. PMID 21149389.
- ↑ 127.0 127.1 "Experimental definition of a clustered regularly interspaced short palindromic duplicon in Escherichia coli". Journal of Molecular Biology 423 (1): 14–16. October 2012. doi:10.1016/j.jmb.2012.06.037. PMID 22771574.
- ↑ 128.0 128.1 128.2 "Molecular memory of prior infections activates the CRISPR/Cas adaptive bacterial immunity system". Nature Communications 3: 945. July 2012. doi:10.1038/ncomms1937. PMID 22781758. Bibcode: 2012NatCo...3..945D.
- ↑ "Recognition and maturation of effector RNAs in a CRISPR interference pathway". Nature Structural & Molecular Biology 18 (6): 688–692. June 2011. doi:10.1038/nsmb.2042. PMID 21572444.
- ↑ "An RNA-induced conformational change required for CRISPR RNA cleavage by the endoribonuclease Cse3". Nature Structural & Molecular Biology 18 (6): 680–687. June 2011. doi:10.1038/nsmb.2043. PMID 21572442.
- ↑ "Sequence- and structure-specific RNA processing by a CRISPR endonuclease". Science 329 (5997): 1355–1358. September 2010. doi:10.1126/science.1192272. PMID 20829488. Bibcode: 2010Sci...329.1355H.
- ↑ 132.0 132.1 "Evolutionary conservation of sequence and secondary structures in CRISPR repeats". Genome Biology 8 (4): R61. 2007. doi:10.1186/gb-2007-8-4-r61. PMID 17442114.
- ↑ "Cas6 is an endoribonuclease that generates guide RNAs for invader defense in prokaryotes". Genes & Development 22 (24): 3489–3496. December 2008. doi:10.1101/gad.1742908. PMID 19141480.
- ↑ "Interaction of the Cas6 riboendonuclease with CRISPR RNAs: recognition and cleavage". Structure 19 (2): 257–264. February 2011. doi:10.1016/j.str.2010.11.014. PMID 21300293.
- ↑ "Evolution of CRISPR RNA recognition and processing by Cas6 endonucleases". Nucleic Acids Research 42 (2): 1341–1353. January 2014. doi:10.1093/nar/gkt922. PMID 24150936.
- ↑ 136.0 136.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid22949671 - ↑ "Interference by clustered regularly interspaced short palindromic repeat (CRISPR) RNA is governed by a seed sequence". Proceedings of the National Academy of Sciences of the United States of America 108 (25): 10098–10103. June 2011. doi:10.1073/pnas.1104144108. PMID 21646539. Bibcode: 2011PNAS..10810098S.
- ↑ "Dynamic properties of the Sulfolobus CRISPR/Cas and CRISPR/Cmr systems when challenged with vector-borne viral and plasmid genes and protospacers". Molecular Microbiology 79 (1): 35–49. January 2011. doi:10.1111/j.1365-2958.2010.07452.x. PMID 21166892.
- ↑ "In vivo activity of CRISPR-mediated virus defence in a hyperthermophilic archaeon". Molecular Microbiology 80 (2): 481–491. April 2011. doi:10.1111/j.1365-2958.2011.07586.x. PMID 21385233.
- ↑ "Structural basis for CRISPR RNA-guided DNA recognition by Cascade". Nature Structural & Molecular Biology 18 (5): 529–536. May 2011. doi:10.1038/nsmb.2019. PMID 21460843. https://pure.rug.nl/ws/files/6761943/2011NatStructMolBiolJoreSupp.pdf.
- ↑ "Structures of the RNA-guided surveillance complex from a bacterial immune system". Nature 477 (7365): 486–489. September 2011. doi:10.1038/nature10402. PMID 21938068. Bibcode: 2011Natur.477..486W.
- ↑ "Structure and mechanism of the CMR complex for CRISPR-mediated antiviral immunity". Molecular Cell 45 (3): 303–313. February 2012. doi:10.1016/j.molcel.2011.12.013. PMID 22227115.
- ↑ 143.0 143.1 "RNA-guided RNA cleavage by a CRISPR RNA-Cas protein complex". Cell 139 (5): 945–956. November 2009. doi:10.1016/j.cell.2009.07.040. PMID 19945378.
- ↑ "RNA-activated DNA cleavage by the Type III-B CRISPR–Cas effector complex". Genes & Development 30 (4): 460–470. 2016. doi:10.1101/gad.273722.115. PMID 26848046.
- ↑ "Co-transcriptional DNA and RNA Cleavage during Type III CRISPR-Cas Immunity". Cell 161 (5): 1164–1174. 2015. doi:10.1016/j.cell.2015.04.027. PMID 25959775.
- ↑ 146.0 146.1 146.2 "Self versus non-self discrimination during CRISPR RNA-directed immunity". Nature 463 (7280): 568–571. January 2010. doi:10.1038/nature08703. PMID 20072129. Bibcode: 2010Natur.463..568M.
- ↑ "Casposons: mobile genetic elements that gave rise to the CRISPR-Cas adaptation machinery". Current Opinion in Microbiology 38: 36–43. August 2017. doi:10.1016/j.mib.2017.04.004. PMID 28472712.
- ↑ "CRISPR-Cas: evolution of an RNA-based adaptive immunity system in prokaryotes". RNA Biology 10 (5): 679–686. May 2013. doi:10.4161/rna.24022. PMID 23439366.
- ↑ "Diversity, classification and evolution of CRISPR-Cas systems". Current Opinion in Microbiology 37: 67–78. June 2017. doi:10.1016/j.mib.2017.05.008. PMID 28605718.
- ↑ 150.0 150.1 "Diversity and evolution of class 2 CRISPR-Cas systems". Nature Reviews. Microbiology 15 (3): 169–182. March 2017. doi:10.1038/nrmicro.2016.184. PMID 28111461.
- ↑ "Probabilistic models for CRISPR spacer content evolution". BMC Evolutionary Biology 13 (1): 54. February 2013. doi:10.1186/1471-2148-13-54. PMID 23442002. Bibcode: 2013BMCEE..13...54K.
- ↑ "Adaptation in CRISPR-Cas Systems". Molecular Cell 61 (6): 797–808. March 2016. doi:10.1016/j.molcel.2016.01.030. PMID 26949040.
- ↑ "Is evolution Darwinian or/and Lamarckian?". Biology Direct 4: 42. November 2009. doi:10.1186/1745-6150-4-42. PMID 19906303.
- ↑ "Lamarckian Illusions". Trends in Ecology & Evolution 30 (10): 566–568. October 2015. doi:10.1016/j.tree.2015.08.003. PMID 26411613.
- ↑ 155.0 155.1 "Just how Lamarckian is CRISPR-Cas immunity: the continuum of evolvability mechanisms". Biology Direct 11 (1): 9. February 2016. doi:10.1186/s13062-016-0111-z. PMID 26912144.
- ↑ "Germ warfare in a microbial mat community: CRISPRs provide insights into the co-evolution of host and viral genomes". PLOS ONE 4 (1): e4169. 2009. doi:10.1371/journal.pone.0004169. PMID 19132092. Bibcode: 2009PLoSO...4.4169H.
- ↑ "A CRISPR/Cas system mediates bacterial innate immune evasion and virulence". Nature 497 (7448): 254–257. May 2013. doi:10.1038/nature12048. PMID 23584588. Bibcode: 2013Natur.497..254S.
- ↑ "The small, slow and specialized CRISPR and anti-CRISPR of Escherichia and Salmonella". PLOS ONE 5 (6): e11126. June 2010. doi:10.1371/journal.pone.0011126. PMID 20559554. Bibcode: 2010PLoSO...511126T.
- ↑ 159.0 159.1 159.2 "Diverse CRISPRs evolving in human microbiomes". PLOS Genetics 8 (6): e1002441. 2012. doi:10.1371/journal.pgen.1002441. PMID 22719260.
- ↑ 160.0 160.1 "Phage mutations in response to CRISPR diversification in a bacterial population". Environmental Microbiology 15 (2): 463–470. February 2013. doi:10.1111/j.1462-2920.2012.02879.x. PMID 23057534. Bibcode: 2013EnvMi..15..463S.
- ↑ "Diversification of CRISPR within coexisting genotypes in a natural population of the bloom-forming cyanobacterium Microcystis aeruginosa". Microbiology 160 (Pt 5): 903–916. May 2014. doi:10.1099/mic.0.073494-0. PMID 24586036.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid24728998 - ↑ "CRISPR—a widespread system that provides acquired resistance against phages in bacteria and archaea". Nature Reviews. Microbiology 6 (3): 181–186. March 2008. doi:10.1038/nrmicro1793. PMID 18157154. https://digital.library.unt.edu/ark:/67531/metadc893045/. "Table 1: Web resources for CRISPR analysis".
- ↑ "Comparisons of clustered regularly interspaced short palindromic repeats and viromes in human saliva reveal bacterial adaptations to salivary viruses". Environmental Microbiology 14 (9): 2564–2576. September 2012. doi:10.1111/j.1462-2920.2012.02775.x. PMID 22583485. Bibcode: 2012EnvMi..14.2564P.
- ↑ "Reassortment of CRISPR repeat-spacer loci in Sulfolobus islandicus". Environmental Microbiology 15 (11): 3065–3076. November 2013. doi:10.1111/1462-2920.12146. PMID 23701169. Bibcode: 2013EnvMi..15.3065H.
- ↑ "CRISPR associated diversity within a population of Sulfolobus islandicus". PLOS ONE 5 (9): e12988. September 2010. doi:10.1371/journal.pone.0012988. PMID 20927396. Bibcode: 2010PLoSO...512988H.
- ↑ 167.0 167.1 167.2 "Virus-borne mini-CRISPR arrays are involved in interviral conflicts". Nature Communications 10 (1): 5204. November 2019. doi:10.1038/s41467-019-13205-2. PMID 31729390. Bibcode: 2019NatCo..10.5204M.
- ↑ "Crass: identification and reconstruction of CRISPR from unassembled metagenomic data". Nucleic Acids Research 41 (10): e105. May 2013. doi:10.1093/nar/gkt183. PMID 23511966.
- ↑ "CRISPR targeting reveals a reservoir of common phages associated with the human gut microbiome". Genome Research 22 (10): 1985–1994. October 2012. doi:10.1101/gr.138297.112. PMID 22732228.
- ↑ "The phage-related chromosomal islands of Gram-positive bacteria". Nature Reviews Microbiology 8 (8): 541–551. August 2010. doi:10.1038/nrmicro2393. PMID 20634809.
- ↑ "Staphylococcal pathogenicity island interference with helper phage reproduction is a paradigm of molecular parasitism". Proceedings of the National Academy of Sciences of the United States of America 109 (40): 16300–16305. October 2012. doi:10.1073/pnas.1204615109. PMID 22991467. Bibcode: 2012PNAS..10916300R.
- ↑ "Precisely modulated pathogenicity island interference with late phage gene transcription". Proceedings of the National Academy of Sciences of the United States of America 111 (40): 14536–14541. October 2014. doi:10.1073/pnas.1406749111. PMID 25246539. Bibcode: 2014PNAS..11114536R.
- ↑ "A bacteriophage encodes its own CRISPR/Cas adaptive response to evade host innate immunity". Nature 494 (7438): 489–491. February 2013. doi:10.1038/nature11927. PMID 23446421. Bibcode: 2013Natur.494..489S.
- ↑ "Bacteriophage ICP1: A Persistent Predator of Vibrio cholerae". Annual Review of Virology 8 (1): 285–304. September 2021. doi:10.1146/annurev-virology-091919-072020. ISSN 2327-056X. PMID 34314595.
- ↑ "What is CRISPR and How does it work?". 30 April 2018. https://www.livescience.tech/2018/04/30/what-is-crispr-how-does-it-work-is-it-gene-editing/.
- ↑ "Current Status and Perspectives on the Application of CRISPR/Cas9 Gene-Editing System to Develop a Low-Gluten, Non-Transgenic Wheat Variety". Foods 10 (2351): 2351. 2 October 2021. doi:10.3390/foods10102351. PMID 34681400.
- ↑ "Genome Editing: Revolutionizing the Crop Improvement". Plant Molecular Biology Reporte 39 (4): 752–772. March 2021. doi:10.1007/s11105-021-01286-7.
- ↑ "Genome editing. The new frontier of genome engineering with CRISPR-Cas9". Science 346 (6213): 1258096. November 2014. doi:10.1126/science.1258096. PMID 25430774.
- ↑ 179.0 179.1 "CRISPR, the disruptor". Nature 522 (7554): 20–24. June 2015. doi:10.1038/522020a. PMID 26040877. Bibcode: 2015Natur.522...20L.
- ↑ "Development and applications of CRISPR-Cas9 for genome engineering". Cell 157 (6): 1262–1278. June 2014. doi:10.1016/j.cell.2014.05.010. PMID 24906146.
- ↑ "Can CRISPR-Cas9 gene drives curb malaria?". Nature Biotechnology 34 (2): 149–150. February 2016. doi:10.1038/nbt.3473. PMID 26849518. https://ora.ox.ac.uk/objects/uuid:e0686ae4-ec7a-4827-a5aa-40b64960beb8.
- ↑ "Assessment of Cas12a-mediated gene editing efficiency in plants". Plant Biotechnology Journal 17 (10): 1971–1984. October 2019. doi:10.1111/pbi.13113. PMID 30950179.
- ↑ "In A 1st, Doctors In U.S. Use CRISPR Tool To Treat Patient With Genetic Disorder". https://www.npr.org/sections/health-shots/2019/07/29/744826505/sickle-cell-patient-reveals-why-she-is-volunteering-for-landmark-gene-editing-st.
- ↑ National Institute on Drug Abuse (2020-02-14). "Antiretroviral Therapy Combined With CRISPR Gene Editing Can Eliminate HIV Infection in Mice" (in en). https://www.drugabuse.gov/news-events/nida-notes/2020/02/antiretroviral-therapy-combined-crispr-gene-editing-can-eliminate-hiv-infection-in-mice.
- ↑ "In A 1st, Scientists Use Revolutionary Gene-Editing Tool To Edit Inside A Patient". https://www.npr.org/sections/health-shots/2020/03/04/811461486/in-a-1st-scientists-use-revolutionary-gene-editing-tool-to-edit-inside-a-patient.
- ↑ The-Crispr (2019-07-15). "Listen Radiolab CRISPR podcast". https://the-crispr.com/listen-radiolab-crispr-podcast/.
- ↑ "CRISPR studies muddy results of older gene research". Nature. 2017. doi:10.1038/nature.2017.21763.
- ↑ Torres-Ruiz, Raul; Rodriguez-Perales, Sandra (January 2017). "CRISPR-Cas9 technology: applications and human disease modelling". Briefings in Functional Genomics 16 (1): 4–12. doi:10.1093/bfgp/elw025. ISSN 2041-2657. PMID 27345434.
- ↑ "CRISPR gene editing in pluripotent stem cells reveals the function of MBNL proteins during human in vitro myogenesis". Human Molecular Genetics 31 (1): 41–56. December 2021. doi:10.1093/hmg/ddab218. PMID 34312665.
- ↑ "CRISPR-Cas9 Mediated Gene Editing: A Revolution in Genome Engineering". Biomarkers and Applications 1 (2). 2017-09-12. doi:10.29011/2576-9588.100111. https://www.gavinpublishers.com/articles/short-commentary/Biomarkers-and-Applications/crispr-cas9-mediated-gene-editing-a-revolution-in-genome-engineering.
- ↑ Huai, Cong; Jia, Chenqiang; Sun, Ruilin; Xu, Peipei; Min, Taishan; Wang, Qihan; Zheng, Chengde; Chen, Hongyan et al. (2017-05-15). "CRISPR/Cas9-mediated somatic and germline gene correction to restore hemostasis in hemophilia B mice". Human Genetics 136 (7): 875–883. doi:10.1007/s00439-017-1801-z. ISSN 0340-6717. PMID 28508290. http://dx.doi.org/10.1007/s00439-017-1801-z.
- ↑ Swiech, Lukasz; Heidenreich, Matthias; Banerjee, Abhishek; Habib, Naomi; Li, Yinqing; Trombetta, John; Sur, Mriganka; Zhang, Feng (2014-10-19). "In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9". Nature Biotechnology 33 (1): 102–106. doi:10.1038/nbt.3055. ISSN 1087-0156. PMID 25326897. PMC 4492112. http://dx.doi.org/10.1038/nbt.3055.
- ↑ Artero-Castro, Ana; Long, Kathleen; Bassett, Andrew; Ávila-Fernandez, Almudena; Cortón, Marta; Vidal-Puig, Antonio; Jendelova, Pavla; Rodriguez-Jimenez, Francisco et al. (2021-02-20). "Gene Correction Recovers Phagocytosis in Retinal Pigment Epithelium Derived from Retinitis Pigmentosa-Human-Induced Pluripotent Stem Cells". International Journal of Molecular Sciences 22 (4): 2092. doi:10.3390/ijms22042092. ISSN 1422-0067. PMID 33672445.
- ↑ Zhu, Haocheng; Li, Chao; Gao, Caixia (2020-09-24). "Applications of CRISPR–Cas in agriculture and plant biotechnology". Nature Reviews Molecular Cell Biology 21 (11): 661–677. doi:10.1038/s41580-020-00288-9. ISSN 1471-0072. PMID 32973356. http://dx.doi.org/10.1038/s41580-020-00288-9.
- ↑ Lu, Kai; Wu, Bowen; Wang, Jie; Zhu, Wei; Nie, Haipeng; Qian, Junjie; Huang, Weiting; Fang, Zhongming (2018-03-25). "Blocking amino acid transporter Os<scp>AAP</scp>3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice". Plant Biotechnology Journal 16 (10): 1710–1722. doi:10.1111/pbi.12907. ISSN 1467-7644.
- ↑ Sánchez‐León, Susana; Gil‐Humanes, Javier; Ozuna, Carmen V.; Giménez, María J.; Sousa, Carolina; Voytas, Daniel F.; Barro, Francisco (2017-11-24). "Low‐gluten, nontransgenic wheat engineered with CRISPR/Cas9". Plant Biotechnology Journal 16 (4): 902–910. doi:10.1111/pbi.12837. ISSN 1467-7644.
- ↑ Wang, Yanpeng; Cheng, Xi; Shan, Qiwei; Zhang, Yi; Liu, Jinxing; Gao, Caixia; Qiu, Jin-Long (2014-07-20). "Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew". Nature Biotechnology 32 (9): 947–951. doi:10.1038/nbt.2969. ISSN 1087-0156. PMID 25038773. http://dx.doi.org/10.1038/nbt.2969.
- ↑ Demirci, Selami; Leonard, Alexis; Haro-Mora, Juan J.; Uchida, Naoya; Tisdale, John F. (2019), "CRISPR/Cas9 for Sickle Cell Disease: Applications, Future Possibilities, and Challenges", Cell Biology and Translational Medicine, Volume 5, Advances in Experimental Medicine and Biology, 1144, Cham: Springer International Publishing, pp. 37–52, doi:10.1007/5584_2018_331, ISBN 978-3-030-17588-7, PMID 30715679, http://dx.doi.org/10.1007/5584_2018_331, retrieved 2023-12-10
- ↑ Fortunato, Fernanda; Rossi, Rachele; Falzarano, Maria Sofia; Ferlini, Alessandra (2021-02-17). "Innovative Therapeutic Approaches for Duchenne Muscular Dystrophy". Journal of Clinical Medicine 10 (4): 820. doi:10.3390/jcm10040820. ISSN 2077-0383. PMID 33671409.
- ↑ Stadtmauer, Edward A.; Fraietta, Joseph A.; Davis, Megan M.; Cohen, Adam D.; Weber, Kristy L.; Lancaster, Eric; Mangan, Patricia A.; Kulikovskaya, Irina et al. (2020-02-28). "CRISPR-engineered T cells in patients with refractory cancer". Science 367 (6481). doi:10.1126/science.aba7365. ISSN 0036-8075. PMID 32029687.
- ↑ Jiang, Fuguo; Doudna, Jennifer A. (2017-05-22). "CRISPR–Cas9 Structures and Mechanisms". Annual Review of Biophysics 46 (1): 505–529. doi:10.1146/annurev-biophys-062215-010822. ISSN 1936-122X.
- ↑ Zhang, Song; Shen, Jiangtao; Li, Dali; Cheng, Yiyun (2021). "Strategies in the delivery of Cas9 ribonucleoprotein for CRISPR/Cas9 genome editing". Theranostics 11 (2): 614–648. doi:10.7150/thno.47007. ISSN 1838-7640. PMID 33391496.
- ↑ 203.0 203.1 "COVID-19: molecular and serological detection methods". PeerJ 8: e10180. October 2020. doi:10.7717/peerj.10180. PMID 33083156.
- ↑ "Simultaneous repression of multiple bacterial genes using nonrepetitive extra-long sgRNA arrays". Nature Biotechnology 37 (11): 1294–1301. November 2019. doi:10.1038/s41587-019-0286-9. PMID 31591552. https://www.osti.gov/biblio/1569832.
- ↑ "Depletion of Abundant Sequences by Hybridization (DASH): using Cas9 to remove unwanted high-abundance species in sequencing libraries and molecular counting applications". Genome Biology 17 (1): 41. March 2016. doi:10.1186/s13059-016-0904-5. PMID 26944702.
- ↑ "CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity". Science 360 (6387): 436–439. April 2018. doi:10.1126/science.aar6245. PMID 29449511. Bibcode: 2018Sci...360..436C.
- ↑ 207.0 207.1 "CRISPR Applications in Plant Bacteriology: today and future perspectives". CRISPR and RNAi Systems: Nanobiotechnology Approaches to Plant Breeding and Protection. Amsterdam: Elsevier. 2021. pp. xxxvi+804. ISBN 978-0-12-821911-9. OCLC 1240283203.
- ↑ "Detection of SARS-CoV-2 with SHERLOCK One-Pot Testing". The New England Journal of Medicine 383 (15): 1492–1494. October 2020. doi:10.1056/NEJMc2026172. PMID 32937062.
- ↑ "Clinical validation of a Cas13-based assay for the detection of SARS-CoV-2 RNA". Nature Biomedical Engineering 4 (12): 1140–1149. December 2020. doi:10.1038/s41551-020-00603-x. PMID 32848209.
- ↑ "Can CRISPR/Cas Technology Be a Felicitous Stratagem Against the COVID-19 Fiasco? Prospects and Hitches". Frontiers in Molecular Biosciences 7: 557377. September 2020. doi:10.3389/fmolb.2020.557377. PMID 33134311.
- ↑ "CRISPR Technology in Gene-Editing-Based Detection and Treatment of SARS-CoV-2". Frontiers in Molecular Biosciences 8: 772788. 2022-01-11. doi:10.3389/fmolb.2021.772788. PMID 35087864.
- ↑ "SHERLOCK: nucleic acid detection with CRISPR nucleases". Nature Protocols 14 (10): 2986–3012. October 2019. doi:10.1038/s41596-019-0210-2. PMID 31548639.
- ↑ "All-in-One Dual CRISPR-Cas12a (AIOD-CRISPR) Assay: A Case for Rapid, Ultrasensitive and Visual Detection of Novel Coronavirus SARS-CoV-2 and HIV virus". bioRxiv. March 2020. doi:10.1101/2020.03.19.998724. PMID 32511323.
Further reading
- CRISPR-Cas: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press. 23 March 2016. ISBN 978-1-62182-131-1.
- "Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems". Science 353 (6299): aad5147. August 2016. doi:10.1126/science.aad5147. PMID 27493190. http://dspace.mit.edu/bitstream/1721.1/113195/1/Zhang5.pdf.
- "CRISPR-Cas systems for editing, regulating and targeting genomes". Nature Biotechnology 32 (4): 347–355. April 2014. doi:10.1038/nbt.2842. PMID 24584096.
- "Rationally engineered Cas9 nucleases with improved specificity". Science 351 (6268): 84–88. January 2016. doi:10.1126/science.aad5227. PMID 26628643. Bibcode: 2016Sci...351...84S.
- "CRISPR-based technologies: prokaryotic defense weapons repurposed". Trends in Genetics 30 (3): 111–118. March 2014. doi:10.1016/j.tig.2014.01.003. PMID 24555991.
- "CRISPR-Cas systems: beyond adaptive immunity". Nature Reviews Microbiology 12 (5): 317–326. May 2014. doi:10.1038/nrmicro3241. PMID 24704746.
- "Virus population dynamics and acquired virus resistance in natural microbial communities". Science 320 (5879): 1047–1050. May 2008. doi:10.1126/science.1157358. PMID 18497291. Bibcode: 2008Sci...320.1047A.
- "Prokaryotic silencing (psi)RNAs in Pyrococcus furiosus". RNA 14 (12): 2572–2579. December 2008. doi:10.1261/rna.1246808. PMID 18971321.
- "Analysis of CRISPR in Streptococcus mutans suggests frequent occurrence of acquired immunity against infection by M102-like bacteriophages". Microbiology 155 (Pt 6): 1966–1976. June 2009. doi:10.1099/mic.0.027508-0. PMID 19383692.
- "RNAi: prokaryotes get in on the act". Cell 139 (5): 863–865. November 2009. doi:10.1016/j.cell.2009.11.018. PMID 19945373.
- "The CRISPR system: small RNA-guided defense in bacteria and archaea". Molecular Cell 37 (1): 7–19. January 2010. doi:10.1016/j.molcel.2009.12.033. PMID 20129051.
- "Identification and characterization of E. coli CRISPR-cas promoters and their silencing by H-NS". Molecular Microbiology 75 (6): 1495–1512. March 2010. doi:10.1111/j.1365-2958.2010.07073.x. PMID 20132443.
- "Diversity of CRISPR loci in Escherichia coli". Microbiology 156 (Pt 5): 1351–1361. May 2010. doi:10.1099/mic.0.036046-0. PMID 20133361.
- "CRISPR/Cas system and its role in phage-bacteria interactions". Annual Review of Microbiology 64: 475–493. 2010. doi:10.1146/annurev.micro.112408.134123. PMID 20528693.
- "CRISPR-Cas: an adaptive immunity system in prokaryotes". F1000 Biology Reports 1: 95. December 2009. doi:10.3410/B1-95. PMID 20556198.
- "The age of the red pen". The Economist. August 22, 2015. ISSN 0013-0613. https://www.economist.com/news/briefing/21661799-it-now-easy-edit-genomes-plants-animals-and-humans-age-red-pen.
- "Genome engineering using the CRISPR-Cas9 system.". Nature Protocols 8 (11): 2281–2308. 2013. doi:10.1038/nprot.2013.143. PMID 24157548.
External links
- "Advanced Gene Editing: CRISPR-Cas9". Congressional Research Service. https://fas.org/sgp/crs/misc/R44824.pdf.
- "Jennifer Doudna talk: Genome Engineering with CRISPR-Cas9: Birth of a Breakthrough Technology". 10 September 2022. https://www.ibiology.org/ibiomagazine/jennifer-doudna-genome-engineering-with-crispr-cas9-birth-of-a-breakthrough-technology.html.
- "Human Nature". NOVA. Season 47. Episode 9. September 9, 2020. PBS. WGBH. Retrieved April 7, 2023.
Protein Data Bank
- Overview of all the structural information available in the PDB for UniProt: Q46901 (CRISPR system Cascade subunit CasA) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: P76632 (CRISPR system Cascade subunit CasB) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q46899 (CRISPR system Cascade subunit CasC) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q46898 (CRISPR system Cascade subunit CasD) at the PDBe-KB.
- Overview of all the structural information available in the PDB for UniProt: Q46897 (CRISPR system Cascade subunit CasE) at the PDBe-KB.
 |