Medicine:Hereditary folate malabsorption
| Hereditary folate malabsorption | |
|---|---|
| Other names | Congenital folate malabsorption |
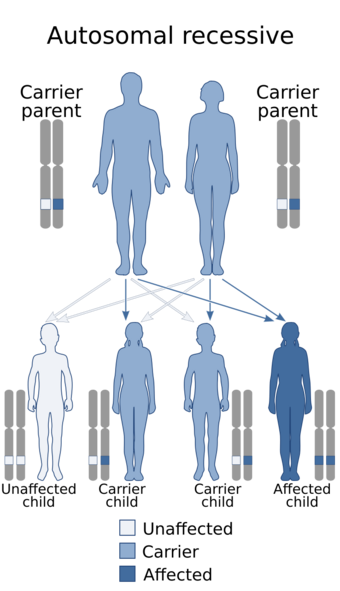 | |
| Hereditary folate malabsorption is inherited in an autosomal recessive manner | |
| Specialty | Medical genetics |
Hereditary folate malabsorption (HFM) is a rare autosomal recessive disorder caused by loss-of-function mutations in the proton-coupled folate transporter (PCFT) gene, resulting in systemic folate deficiency and impaired delivery of folate to the brain.
Signs and symptoms
Affected infants present within a few months after birth with failure to thrive and severe folate deficiency manifested as macrocytic anemia and developmental delays. There can be (i) pancytopenia, (ii) diarrhea and/or mucositis and/or (iii) immune deficiency due to T-cell dysfunction and hypoimmunoglobulinemia resulting in pneumonia usually due to Pneumocystis jirovecii.[1] Recently, several infants with the immune deficiency syndrome were described.[2][3][4] Untreated, or with inadequate treatment, there are progressive systemic and neurological signs with a spectrum of manifestations including seizures that are often intractable. Females with HFM are fertile and, if folate sufficient during pregnancy, have normal offspring. Subjects that carry one mutated PCFT allele are normal. The genomic and clinical features of HFM were recently reviewed.[1][4][5]
Pathophysiology
Extensive clinical studies established that HFM is due to (i) impaired intestinal absorption of folates and (ii) impaired transport of folates across the blood-choroid plexus-cerebrospinal fluid (CSF) barrier.[1][6][7][8] Hence, patients with HFM have very low or undetectable folate blood levels. When a modest dose of a folate is given by mouth, there is impaired intestinal folate absorption without other signs of malabsorption.[citation needed]
Molecular pathogenesis
The molecular basis for HFM was established in 2006 with the identification of the proton-coupled folate transporter (PCFT) as the mechanism of intestinal absorption of folates and the detection of loss-of-function mutations in this transporter in subjects with a clinical diagnosis of HFM.[9][10] Hence, beyond the characteristic clinical features, genotyping is now available to establish the diagnosis of HFM.[citation needed]
PCFT
PCFT is located on chromosome 17q11.2 and consists of 459 amino acids, with five exons, and a MW of approximately 50kDa.[9] The secondary structure has been established and consists of twelve transmembrane domains with the N- and C- termini directed into the cytoplasm.[11][12] The properties of this transporter and its physiological and pharmacological roles were recently reviewed.[13][14] Elements of PCFT regulation have been described and include the minimal promoter,[15][16] Vitamin D[17] and NRF1[18] response elements. PCFT operates most efficiently when there is a strong transmembrane pH gradient. Under these conditions transport of a folate molecule across the cell membrane is accompanied by a sufficient number of protons to result a positive charge and current mediated by the ternary carrier complex.[9][19] It is the pH gradient present across the apical brush-border membrane of the proximal jejunum,[20] where PCFT is highly expressed, that drives intestinal folate absorption.[20][21] PCFT is expressed to a lesser extent elsewhere in the small and large intestine along with the canalicular membrane of the hepatic sinusoid and in the apical brush-border membrane of the proximal tubule of the kidney. However, its function at these latter sites is unclear.[22] As indicated above, PCFT is also expressed at the basolateral membrane of ependymal cells of the choroid plexus where it presumably plays a role in transport of folates into the CSF.[22]
Diagnosis
The CSF folate level is usually undetectable at the time of diagnosis. Even when the blood folate level is corrected, or far above normal, the CSF folate level remains low, consistent with impaired transport across the choroid plexus. The normal CSF folate level in children over the first three years of life is in the 75 to 150 nM range.[23][24] In subjects with HFM it is very difficult indeed, rarely possible, to bring the CSF folate level into the normal range even with substantial doses of parenteral folate[25]
Differential diagnosis
HFM must be distinguished from cerebral folate deficiency (CFD)– a syndrome in which there is normal intestinal folate absorption, without systemic folate deficiency, but a decrease in CSF folate levels. This can accompany a variety of disorders.[26] One form of CFD is due to loss-of-mutations in folate receptor-α, (FRα), which transports folates via an endocytic process.[27][28][29] While PCFT is expressed primarily at the basolateral membrane of the choroid plexus, FRα is expressed primarily at the apical brush-border membrane.[30] Unlike subjects with HFM, patients with CFD present with neurological signs a few years after birth. The basis for the delay in the appearance of clinical manifestations due to loss of FRα function is not clear; the normal blood folate levels may be protective, although for a limited time.
Treatment
Because HFM is a rare disorder, there are no studies that define its optimal treatment. Correction of the systemic folate deficiency, with the normalization of folate blood levels, is easily achieved with high doses of oral folates or much smaller doses of parenteral folate.[1][6][7][8] This will rapidly correct the anemia, immune deficiency and GI signs. The challenge is to achieve adequate treatment of the neurological component of HFM. It is essential that the folate dose is sufficiently high to achieve CSF folate levels as close as possible to the normal range for the age of the child.[23][24] This requires close monitoring of the CSF folate level.[25] The physiological folate is 5-methyltetrahydrofolate but the oral formulation available is insufficient for treatment of this disorder and a parenteral form is not available. The optimal folate at this time is 5-formyltetrahydrofolate which, after administration, is converted to 5-methyltetrahydrofolate. The racemic mixture of 5-formyltetrahydrofolate (leucovorin) is generally available; the active S-isomer, levoleucovorin, may be obtained as well. Parenteral administration is the optimal treatment if that is possible. Folic acid should not be used for the treatment of HFM. Folic acid is not a physiological folate. It binds tightly to, and may impede, FRα-mediated endocytosis which plays an important role in the transport of folates across the choroid plexus into the CSF (see above).[30][22] For a further consideration of treatment see GeneReviews.[5]
Epidemiology
As of June 2014 (the latest update on HFM[5] in GeneReviews) a total of 32 families had been reported with a clinical diagnosis of HFM of which there was genotypic confirmation in 24 families. Since then, another two confirmed cases have been reported[3][4] and an additional case was reported based on a clinical diagnosis alone.[31] Most cases emerge from consanguineous parents with homozygous mutations. There are three instances of HFM from non-consanguineous parents in which there were heterozygous mutations. HFM cases are worldwide with mostly private mutations. However, a number of families of Puerto Rican ancestry have been reported with a common pathogenic variant at a splice receptor site resulting in the deletion of exon 3 and the absence of transport function.[2][5][9][32] A subsequent population-based study of newborn infants in Puerto Rico identified the presence of the same variant on the island.[33] Most of the pathogenic variants result in a complete loss of the PCFT protein or point mutations that result in the complete loss of function. However, residual function can be detected with some of the point mutants.[34]
Figures

See also
References
- ↑ 1.0 1.1 1.2 1.3 Geller, J; Kronn, D; Jayabose, S; Sandoval, C (January 2002). "Hereditary folate malabsorption: family report and review of the literature.". Medicine 81 (1): 51–68. doi:10.1097/00005792-200201000-00004. PMID 11807405.
- ↑ 2.0 2.1 Borzutzky, A; Crompton, B; Bergmann, AK; Giliani, S; Baxi, S; Martin, M; Neufeld, EJ; Notarangelo, LD (December 2009). "Reversible severe combined immunodeficiency phenotype secondary to a mutation of the proton-coupled folate transporter.". Clinical Immunology (Orlando, Fla.) 133 (3): 287–94. doi:10.1016/j.clim.2009.08.006. PMID 19740703.
- ↑ 3.0 3.1 Kishimoto, K; Kobayashi, R; Sano, H; Suzuki, D; Maruoka, H; Yasuda, K; Chida, N; Yamada, M et al. (July 2014). "Impact of folate therapy on combined immunodeficiency secondary to hereditary folate malabsorption.". Clinical Immunology (Orlando, Fla.) 153 (1): 17–22. doi:10.1016/j.clim.2014.03.014. PMID 24691418.
- ↑ 4.0 4.1 4.2 Erlacher, M; Grünert, SC; Cseh, A; Steinfeld, R; Salzer, U; Lausch, E; Nosswitz, U; Dückers, G et al. (June 2015). "Reversible pancytopenia and immunodeficiency in a patient with hereditary folate malabsorption.". Pediatric Blood & Cancer 62 (6): 1091–4. doi:10.1002/pbc.25364. PMID 25504888.
- ↑ 5.0 5.1 5.2 5.3 Diop-Bove, N; Kronn, D; Goldman, ID; Pagon, RA; Adam, MP; Ardinger, HH; Wallace, SE; Amemiya, A et al. (1993). Hereditary Folate Malabsorption. PMID 20301716.
- ↑ 6.0 6.1 Lanzkowsky, P; Erlandson, ME; Bezan, AI (October 1969). "Isolated defect of folic acid absorption associated with mental retardation and cerebral calcification.". Blood 34 (4): 452–65. doi:10.1182/blood.V34.4.452.452. PMID 4980683.
- ↑ 7.0 7.1 Poncz, M; Colman, N; Herbert, V; Schwartz, E; Cohen, AR (January 1981). "Therapy of congenital folate malabsorption.". The Journal of Pediatrics 98 (1): 76–9. doi:10.1016/s0022-3476(81)80541-0. PMID 6969796.
- ↑ 8.0 8.1 Urbach, J; Abrahamov, A; Grossowicz, N (January 1987). "Congenital isolated folic acid malabsorption.". Archives of Disease in Childhood 62 (1): 78–80. doi:10.1136/adc.62.1.78. PMID 3813642.
- ↑ 9.0 9.1 9.2 9.3 Qiu, A; Jansen, M; Sakaris, A; Min, SH; Chattopadhyay, S; Tsai, E; Sandoval, C; Zhao, R et al. (1 December 2006). "Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption.". Cell 127 (5): 917–28. doi:10.1016/j.cell.2006.09.041. PMID 17129779.
- ↑ Zhao, R; Min, SH; Qiu, A; Sakaris, A; Goldberg, GL; Sandoval, C; Malatack, JJ; Rosenblatt, DS et al. (15 August 2007). "The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption.". Blood 110 (4): 1147–52. doi:10.1182/blood-2007-02-077099. PMID 17446347.
- ↑ Duddempudi, PK; Goyal, R; Date, SS; Jansen, M (2013). "Delineating the extracellular water-accessible surface of the proton-coupled folate transporter.". PLOS ONE 8 (10): e78301. doi:10.1371/journal.pone.0078301. PMID 24205192. Bibcode: 2013PLoSO...878301D.
- ↑ Zhao, R; Unal, ES; Shin, DS; Goldman, ID (6 April 2010). "Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method.". Biochemistry 49 (13): 2925–31. doi:10.1021/bi9021439. PMID 20225891.
- ↑ Zhao, R; Goldman, ID (2013). "Folate and thiamine transporters mediated by facilitative carriers (SLC19A1-3 and SLC46A1) and folate receptors.". Molecular Aspects of Medicine 34 (2–3): 373–85. doi:10.1016/j.mam.2012.07.006. PMID 23506878.
- ↑ Desmoulin, SK; Hou, Z; Gangjee, A; Matherly, LH (December 2012). "The human proton-coupled folate transporter: Biology and therapeutic applications to cancer.". Cancer Biology & Therapy 13 (14): 1355–73. doi:10.4161/cbt.22020. PMID 22954694.
- ↑ Stark, M; Gonen, N; Assaraf, YG (9 October 2009). "Functional elements in the minimal promoter of the human proton-coupled folate transporter.". Biochemical and Biophysical Research Communications 388 (1): 79–85. doi:10.1016/j.bbrc.2009.07.116. PMID 19643086.
- ↑ Diop-Bove, NK; Wu, J; Zhao, R; Locker, J; Goldman, ID (August 2009). "Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line.". Molecular Cancer Therapeutics 8 (8): 2424–31. doi:10.1158/1535-7163.mct-08-0938. PMID 19671745.
- ↑ Eloranta, JJ; Zaïr, ZM; Hiller, C; Häusler, S; Stieger, B; Kullak-Ublick, GA (November 2009). "Vitamin D3 and its nuclear receptor increase the expression and activity of the human proton-coupled folate transporter.". Molecular Pharmacology 76 (5): 1062–71. doi:10.1124/mol.109.055392. PMID 19666701.
- ↑ Gonen, N; Assaraf, YG (29 October 2010). "The obligatory intestinal folate transporter PCFT (SLC46A1) is regulated by nuclear respiratory factor 1.". The Journal of Biological Chemistry 285 (44): 33602–13. doi:10.1074/jbc.m110.135640. PMID 20724482.
- ↑ Umapathy, NS; Gnana-Prakasam, JP; Martin, PM; Mysona, B; Dun, Y; Smith, SB; Ganapathy, V; Prasad, PD (November 2007). "Cloning and functional characterization of the proton-coupled electrogenic folate transporter and analysis of its expression in retinal cell types.". Investigative Ophthalmology & Visual Science 48 (11): 5299–305. doi:10.1167/iovs.07-0288. PMID 17962486.
- ↑ 20.0 20.1 Said, HM; Smith, R; Redha, R (November 1987). "Studies on the intestinal surface acid microclimate: developmental aspects.". Pediatric Research 22 (5): 497–9. doi:10.1203/00006450-198711000-00002. PMID 3684377.
- ↑ Visentin, M; Diop-Bove, N; Zhao, R; Goldman, ID (2014). "The intestinal absorption of folates.". Annual Review of Physiology 76: 251–74. doi:10.1146/annurev-physiol-020911-153251. PMID 24512081.
- ↑ 22.0 22.1 22.2 Zhao, R; Diop-Bove, N; Visentin, M; Goldman, ID (21 August 2011). "Mechanisms of membrane transport of folates into cells and across epithelia.". Annual Review of Nutrition 31: 177–201. doi:10.1146/annurev-nutr-072610-145133. PMID 21568705.
- ↑ 23.0 23.1 Ormazabal, A; García-Cazorla, A; Pérez-Dueñas, B; Gonzalez, V; Fernández-Alvarez, E; Pineda, M; Campistol, J; Artuch, R (September 2006). "Determination of 5-methyltetrahydrofolate in cerebrospinal fluid of paediatric patients: reference values for a paediatric population.". Clinica Chimica Acta 371 (1–2): 159–62. doi:10.1016/j.cca.2006.03.004. PMID 16624264.
- ↑ 24.0 24.1 Verbeek, MM; Blom, AM; Wevers, RA; Lagerwerf, AJ; van de Geer, J; Willemsen, MA (November 2008). "Technical and biochemical factors affecting cerebrospinal fluid 5-MTHF, biopterin and neopterin concentrations.". Molecular Genetics and Metabolism 95 (3): 127–32. doi:10.1016/j.ymgme.2008.07.004. PMID 18722797.
- ↑ 25.0 25.1 Torres, A; Newton, SA; Crompton, B; Borzutzky, A; Neufeld, EJ; Notarangelo, L; Berry, GT (26 May 2015). CSF 5-Methyltetrahydrofolate Serial Monitoring to Guide Treatment of Congenital Folate Malabsorption Due to Proton-Coupled Folate Transporter (PCFT) Deficiency.. 24. 91–6. doi:10.1007/8904_2015_445. ISBN 978-3-662-48226-1.
- ↑ Hyland, K; Shoffner, J; Heales, SJ (October 2010). "Cerebral folate deficiency.". Journal of Inherited Metabolic Disease 33 (5): 563–70. doi:10.1007/s10545-010-9159-6. PMID 20668945.
- ↑ Steinfeld, R; Grapp, M; Kraetzner, R; Dreha-Kulaczewski, S; Helms, G; Dechent, P; Wevers, R; Grosso, S et al. (September 2009). "Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism.". American Journal of Human Genetics 85 (3): 354–63. doi:10.1016/j.ajhg.2009.08.005. PMID 19732866.
- ↑ Grapp, M; Just, IA; Linnankivi, T; Wolf, P; Lücke, T; Häusler, M; Gärtner, J; Steinfeld, R (July 2012). "Molecular characterization of folate receptor 1 mutations delineates cerebral folate transport deficiency.". Brain: A Journal of Neurology 135 (Pt 7): 2022–31. doi:10.1093/brain/aws122. PMID 22586289.
- ↑ Toelle, SP; Wille, D; Schmitt, B; Scheer, I; Thöny, B; Plecko, B (March 2014). "Sensory stimulus-sensitive drop attacks and basal ganglia calcification: new findings in a patient with FOLR1 deficiency.". Epileptic Disorders 16 (1): 88–92. doi:10.1684/epd.2014.0629. PMID 24556562.
- ↑ 30.0 30.1 Grapp, M; Wrede, A; Schweizer, M; Hüwel, S; Galla, HJ; Snaidero, N; Simons, M; Bückers, J et al. (2013). "Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma.". Nature Communications 4: 2123. doi:10.1038/ncomms3123. PMID 23828504. Bibcode: 2013NatCo...4.2123G.
- ↑ Ahmad, I; Mukhtar, G; Iqbal, J; Ali, SW (January 2015). "Hereditary folate malabsorption with extensive intracranial calcification.". Indian Pediatrics 52 (1): 67–8. doi:10.1007/s13312-015-0571-8. PMID 25638192.
- ↑ Santiago-Borrero, PJ; Santini R, Jr; Pérez-Santiago, E; Maldonado, N (March 1973). "Congenital isolated defect of folic acid absorption.". The Journal of Pediatrics 82 (3): 450–5. doi:10.1016/s0022-3476(73)80119-2. PMID 4540608.
- ↑ Mahadeo, KM; Diop-Bove, N; Ramirez, SI; Cadilla, CL; Rivera, E; Martin, M; Lerner, NB; DiAntonio, L et al. (October 2011). "Prevalence of a loss-of-function mutation in the proton-coupled folate transporter gene (PCFT-SLC46A1) causing hereditary folate malabsorption in Puerto Rico.". The Journal of Pediatrics 159 (4): 623–7.e1. doi:10.1016/j.jpeds.2011.03.005. PMID 21489556.
- ↑ Mahadeo, K; Diop-Bove, N; Shin, D; Unal, ES; Teo, J; Zhao, R; Chang, MH; Fulterer, A et al. (November 2010). "Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption.". American Journal of Physiology. Cell Physiology 299 (5): C1153–61. doi:10.1152/ajpcell.00113.2010. PMID 20686069.
- ↑ Sakurai, Y., Toriumi, N., Sarashina, T. et al. An infantile case of hereditary folate malabsorption with sudden development of pulmonary hemorrhage: a case report. J Med Case Reports 16, 268 (2022). https://doi.org/10.1186/s13256-022-03448-x
External links
| Classification | |
|---|---|
| External resources |
- Hereditary Folate Malabsorbtion at GeneReviews
 |

