Medicine:Intrauterine hypoxia
| Intrauterine hypoxia | |
|---|---|
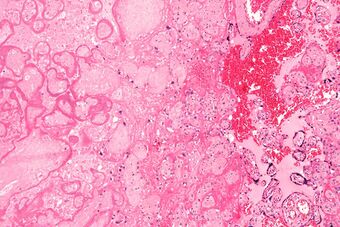 | |
| Micrograph of a placental infarct (left of image), a cause of intrauterine hypoxia. H&E stain. |
Intrauterine hypoxia (also known as fetal hypoxia) occurs when the fetus is deprived of an adequate supply of oxygen. It may be due to a variety of reasons such as prolapse or occlusion of the umbilical cord, placental infarction, maternal diabetes (prepregnancy or gestational diabetes)[1] and maternal smoking. Intrauterine growth restriction may cause or be the result of hypoxia. Intrauterine hypoxia can cause cellular damage that occurs within the central nervous system (the brain and spinal cord). This results in an increased mortality rate, including an increased risk of sudden infant death syndrome (SIDS). Oxygen deprivation in the fetus and neonate have been implicated as either a primary or as a contributing risk factor in numerous neurological and neuropsychiatric disorders such as epilepsy, attention deficit hyperactivity disorder, eating disorders and cerebral palsy.[2][3][4][5][6][7]
Presentation
Maternal Consequences
Complications arising from intrauterine hypoxia are some of most common causes of preeclampsia.[8] Preeclampsia is a hypertensive disorder that occurs during the second trimester (after the 20th week of pregnancy) resulting from a poorly perfused placenta.[9] The World Health Organization estimates that preeclampsia and eclampsia are responsible for about 14% of maternal deaths globally (around 50,000 to 75,000 deaths annually).[10]
During pregnancy, women with preeclampsia faces serious risk of damage to vital organs such as the kidneys, liver, brain, and the blood system. This hypertensive disorder may also cause damage to the placenta leading to issues such as premature births, miscarriages, placental abruption, or even stillbirths. In some cases, preeclampsia can eventually lead to stroke and organ failure. Untreated, preeclampsia can progress and turn into eclampsia which is much more severe with the addition of seizures. Eclampsia seizures could lead to uncontrollable twitching and a loss of consciousness, which could potentially lead to the death of the mother and or the baby.[11]
Cause
Intrauterine hypoxia can be attributed to maternal, placental, or fetal conditions.[12] Kingdom and Kaufmann classifies three categories for the origin of fetal hypoxia: 1) pre-placental (both mother and fetus are hypoxic), 2) utero-placental (mother is normal but placenta and fetus is hypoxic), 3) post-placental (only fetus is hypoxic).[13]
Pre-placental hypoxia is most commonly caused by external hypoxic environments (such as high altitude). It can also be caused by maternal respiratory conditions (such as asthma), cardiovascular conditions (such as heart failure, pulmonary hypertension, and cyanotic heart disease), and hematological conditions (such as anemia).[14] Conditions such as obesity, nutritional deficiencies, infections, chronic inflammations, and stress can also affect the maternal oxygen supply and fetal uptake.[12]
The most preventable cause is maternal smoking. Cigarette smoking by expectant mothers has been shown to have a wide variety of deleterious effects on the developing fetus.[15] Among the negative effects are carbon monoxide induced tissue hypoxia and placental insufficiency which causes a reduction in blood flow from the uterus to the placenta thereby reducing the availability of oxygenated blood to the fetus. Placental insufficiency as a result of smoking has been shown to have a causal effect in the development of pre-eclampsia. While some previous studies have suggested that carbon monoxide from cigarette smoke may have a protective effect against preeclampsia, a recent study conducted by the Genetics of Pre-Eclampsia Consortium in the United Kingdom found that smokers were five times more likely to develop pre-eclampsia.[16] Nicotine alone has been shown to be a teratogen which affects the autonomic nervous system, leading to increased susceptibility to hypoxia-induced brain damage.[16][17][18][19][20][21] Maternal anemia in which smoking has also been implicated is another factor associated with IH/BA.[clarification needed] Smoking by expectant mothers causes a decrease in maternal nucleated red blood cells, thereby reducing the amount of red blood cells available for oxygen transport.[22][23][24]
Utero-placental hypoxia is associated with abnormal placental implantation, impaired vascular remodeling and vascular diseases.[14] It is also associated with pregnancies complicated by gestational hypertension, intrauterine growth restriction, and pre-eclampsia.[25][26]
Post-placental hypoxia is associated with mechanical obstructions of the umbilical cords, reduced uterine artery flow, progressive fetal cardiac failure, and genetic anomalies.[12][14]
The perinatal brain injury occurring as a result of birth asphyxia, manifesting within 48 hours of birth, is a form of hypoxic ischemic encephalopathy.[27]
Diagnosis
Treatment
Treatment of infants with birth asphyxia by lowering the core body temperature is now known to be an effective therapy to reduce mortality and improve neurological outcome in survivors, and hypothermia therapy for neonatal encephalopathy begun within 6 hours of birth significantly increases the chance of normal survival in affected infants.[28]
There has long been a debate over whether newborn infants with birth asphyxia should be resuscitated with 100% oxygen or normal air.[29] It has been demonstrated that high concentrations of oxygen lead to generation of oxygen free radicals, which have a role in reperfusion injury after asphyxia.[30] Research by Ola Didrik Saugstad and others led to new international guidelines on newborn resuscitation in 2010, recommending the use of normal air instead of 100% oxygen.[31][32] Increasing the oxygen concentration to the mother has shown little effect on the fetus as hyperoxygenated blood does not perfuse the placental exchange site well.[33]
Underlying etiology of intrauterine hypoxia serves as a potential therapeutic target. If maternal preeclampsia[34] is the underlying cause of fetal growth restriction (FGR) antihypertensive therapy and magnesium sulfate are potential therapies.[12] Antihypertensive treatment is used to reduce blood pressure and prevent pulmonary edema and cerebral hemorrhages. An effective course of antihypertensive treatments should reduce blood pressure to below 160/110 mmHg. Magnesium sulfate acts as a vasodilator, reducing vascular resistance and protect the blood-brain barrier (BBB). The goal of these treatments is to prolong pregnancy and increase fetal survival. Each day gained by treatment in utero increases fetal survival and intact survival by 1%–2% up to 28 weeks gestation.[35]
Prevention
Medical testing and care can be performed in order to prevent intrauterine hypoxia, though can be difficult. These tests don't directly detect hypoxia, but instead detects the general well-being of the baby and ensures that the baby is healthy since hypoxia causes a wide range of responses. These tests can include prenatal testing, such as fetal movement and amniotic fluid levels, Doppler examination, or fetal heart rate.[36] Another risk factor is premature birth in which medical intervention, such as premature birth prevention or C-section delivery, can be used as prevention for intrauterine hypoxia.[37]
Studies have shown a connection between tetrahydrobiopterin (BH4) deficiency and hypoxia-ischemia brain injury, though further studies need to be done.[38] Measuring fetal BH4 levels can be another way to look out for intrauterine hypoxia.[citation needed]
During birth, birth asphyxia can occur in which cardiotocograph can be used to monitor the baby's health during labor.[39]
Epidemiology
In the United States, intrauterine hypoxia and birth asphyxia were listed together as the tenth leading cause of neonatal death.[40]
Society
IH/BA is also a causative factor in cardiac and circulatory birth defects the sixth most expensive condition, as well as premature birth and low birth weight the second most expensive and it is one of the contributing factors to infant respiratory distress syndrome (RDS) also known as hyaline membrane disease, the most expensive medical condition to treat and the number one cause of infant mortality.[41][42][43]
| Most expensive medical condition treated in U.S. hospitals. 4 out of 10 linked to intrauterine hypoxia/birth asphxia | Cost | Hospital Charge |
| 1. Infant respiratory distress syndrome | $45,542 | $138,224 |
| 2. Premature birth and low birth weight | $44,490 | $119,389 |
| 6. Cardiac and circulatory birth defects | $35,960 | $101,412 |
| 9. Intrauterine hypoxia or birth asphyxia | $27,962 | $74,942 |
Medicolegal
In the United States the National Practitioner Data Bank 2006 Annual Report obstetrics-related cases accounted for 8.7 percent of all 2006 physician Malpractice Payment Reports and had the highest median payment amounts ($333,334).[44]
References
- ↑ Tarvonen M, Hovi P, Sainio S, Vuorela P, Andersson S, Teramo K (2021). "Intrapartal cardiotocographic patterns and hypoxia-related perinatal outcomes in pregnancies complicated by gestational diabetes mellitus". Acta Diabetologica 58 (11): 1563–1573. doi:10.1007/s00592-021-01756-0. PMID 34151398.
- ↑ "The effects of ante- and postnatal hypoxia on the central nervous system and their correction with peptide hormones". Neuroscience and Behavioral Physiology 33 (6): 607–11. July 2003. doi:10.1023/A:1023938905744. PMID 14552554.
- ↑ "[Intrauterine hypoxia and sudden infant death syndrome]". Acta Medica Croatica 56 (3): 109–18. 2002. PMID 12630342.
- ↑ "Chronic fetal hypoxia and sudden infant death syndrome: interaction between maternal smoking and low hematocrit during pregnancy". Pediatrics 86 (4): 535–40. October 1990. doi:10.1542/peds.86.4.535. PMID 2216618.
- ↑ "Intrauterine growth restriction: identification and management". American Family Physician 58 (2): 453–60, 466–7. August 1998. PMID 9713399.
- ↑ "The IUGR newborn". Seminars in Perinatology 32 (3): 219–24. June 2008. doi:10.1053/j.semperi.2007.11.003. PMID 18482625.
- ↑ "Does perinatal asphyxia impair cognitive function without cerebral palsy?". Archives of Disease in Childhood. Fetal and Neonatal Edition 91 (6): F454-9. November 2006. doi:10.1136/adc.2005.092445. PMID 17056843.
- ↑ "Intrauterine hypoxia: clinical consequences and therapeutic perspectives". Research and Reports in Neonatology 5: 79–89. September 2015. doi:10.2147/RRN.S57990.
- ↑ Publishing, Harvard Health (26 October 2018). "Preeclampsia And Eclampsia". https://www.health.harvard.edu/a_to_z/preeclampsia-and-eclampsia-a-to-z.
- ↑ "Global causes of maternal death: a WHO analysis". The Lancet Global Health 2 (6): e323–33. June 2014. doi:10.1016/S2214-109X(14)70227-X. PMID 25103301.
- ↑ "Pre-Eclampsia and Eclampsia: An Update on the Pharmacological Treatment Applied in Portugal". Journal of Cardiovascular Development and Disease 5 (1): 3. January 2018. doi:10.3390/jcdd5010003. PMID 29367581.
- ↑ 12.0 12.1 12.2 12.3 "Hypoxia: From Placental Development to Fetal Programming". Birth Defects Research 109 (17): 1377–1385. October 2017. doi:10.1002/bdr2.1142. PMID 29105382.
- ↑ "Oxygen and placental villous development: origins of fetal hypoxia". Placenta 18 (8): 613–21; discussion 623–6. November 1997. doi:10.1016/s0143-4004(97)90000-x. PMID 9364596.
- ↑ 14.0 14.1 14.2 "Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review". International Journal of Pediatrics 2010: 401323. 2010. doi:10.1155/2010/401323. PMID 20981293.
- ↑ "Smoking and pregnancy--a review on the first major environmental risk factor of the unborn". International Journal of Environmental Research and Public Health 10 (12): 6485–99. November 2013. doi:10.3390/ijerph10126485. PMID 24351784.
- ↑ 16.0 16.1 "Smoking in moderate/severe preeclampsia worsens pregnancy outcome, but smoking cessation limits the damage". Hypertension 51 (4): 1042–6. April 2008. doi:10.1161/HYPERTENSIONAHA.107.106559. PMID 18259022.
- ↑ "Fetal nicotine or cocaine exposure: which one is worse?". The Journal of Pharmacology and Experimental Therapeutics 285 (3): 931–45. June 1998. PMID 9618392.
- ↑ "Maternal smoking is associated with mitochondrial DNA depletion and respiratory chain complex III deficiency in placenta". American Journal of Physiology. Endocrinology and Metabolism 288 (1): E171-7. January 2005. doi:10.1152/ajpendo.00260.2003. PMID 15585597.
- ↑ "[Risk of iugr syndrome development during preeclampsia of the pregnant]". Georgian Medical News (128): 15–7. November 2005. PMID 16369054.
- ↑ "Intrauterine growth restriction in infants of less than thirty-two weeks' gestation: associated placental pathologic features". American Journal of Obstetrics and Gynecology 173 (4): 1049–57. October 1995. doi:10.1016/0002-9378(95)91325-4. PMID 7485292.
- ↑ "Oxygen and placental villous development: origins of fetal hypoxia". Placenta 18 (8): 613–21; discussion 623–6. November 1997. doi:10.1016/S0143-4004(97)90000-X. PMID 9364596.
- ↑ "Effect of maternal smoking on some markers of iron status in umbilical cord blood". Roczniki Akademii Medycznej W Bialymstoku 47: 235–40. 2002. PMID 12533965.
- ↑ "Fetal tobacco syndrome and perinatal outcome". Fetal Diagnosis and Therapy 17 (6): 367–71. 2002. doi:10.1159/000065387. PMID 12393968.
- ↑ Benirschke, Kurt; Kaufmann, Peter (March 2000). Pathology of the human placenta (4th ed.). Springer. pp. 453. ISBN 978-0-387-98894-8.
- ↑ Hutter, Damian; Kingdom, John; Jaeggi, Edgar (2010). "Causes and Mechanisms of Intrauterine Hypoxia and Its Impact on the Fetal Cardiovascular System: A Review". International Journal of Pediatrics 2010: 401323. doi:10.1155/2010/401323. ISSN 1687-9740. PMID 20981293.
- ↑ Keyes, Linda E.; Armaza, J. Fernando; Niermeyer, Susan; Vargas, Enrique; Young, David A.; Moore, Lorna G. (July 2003). "Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia". Pediatric Research 54 (1): 20–25. doi:10.1203/01.PDR.0000069846.64389.DC. ISSN 0031-3998. PMID 12700368.
- ↑ Hypoxic-Ischemic Encephalopathy: Practice Essentials, Background, Pathophysiology. 2019-11-13. https://emedicine.medscape.com/article/973501-overview.
- ↑ Shankaran, Seetha (December 2012). "Therapeutic Hypothermia for Neonatal Encephalopathy". Current Treatment Options in Neurology 14 (6): 608–619. doi:10.1007/s11940-012-0200-y. ISSN 1092-8480. PMID 23007949.
- ↑ "Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis". Lancet 364 (9442): 1329–33. 2004. doi:10.1016/S0140-6736(04)17189-4. PMID 15474135.
- ↑ "Hydrogen peroxide production in leukocytes during cerebral hypoxia and reoxygenation with 100% or 21% oxygen in newborn piglets". Pediatric Research 49 (6): 834–42. June 2001. doi:10.1203/00006450-200106000-00020. PMID 11385146.
- ↑ ILCOR Neonatal Resuscitation Guidelines 2010
- ↑ "Norwegian paediatrician honoured by University of Athens". Norway.gr. http://www.norway.gr/News_and_events/Events/Older-articles/Norwegian-paediatrician-honoured-by-University-of-Athens-/.
- ↑ "Prevention and correction of fetal acidosis and hypoxia". Clinical Obstetrics and Gynecology 17 (3): 115–34. September 1974. doi:10.1097/00003081-197409000-00008. PMID 4606933.
- ↑ "Maternal preeclampsia". Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/preeclampsia/symptoms-causes/syc-20355745.
- ↑ "Predictors of neonatal outcome in early-onset placental dysfunction". Obstetrics and Gynecology 109 (2 Pt 1): 253–61. February 2007. doi:10.1097/01.AOG.0000253215.79121.75. PMID 17267821.
- ↑ "Fetal cerebrovascular response to chronic hypoxia--implications for the prevention of brain damage". The Journal of Maternal-Fetal & Neonatal Medicine 19 (7): 387–96. July 2006. doi:10.1080/14767050600637861. PMID 16923693. http://medlib.mef.hr/655/1/salihagic-kadic_a_et_al_rep_655.pdf.
- ↑ "Preventing Hypoxic-Ischemic Encephalopathy (HIE)" (in en-US). https://hiehelpcenter.org/medical/prevention/.
- ↑ "Tetrahydrobiopterin in the prevention of hypertonia in hypoxic fetal brain". Annals of Neurology 66 (3): 323–31. September 2009. doi:10.1002/ana.21738. PMID 19798726.
- ↑ "Prevention of birth asphyxia: responding appropriately to cardiotocograph (CTG) traces". Best Practice & Research. Clinical Obstetrics & Gynaecology. Risk Management in Obstetrics and Gynaecology 21 (4): 609–24. August 2007. doi:10.1016/j.bpobgyn.2007.02.008. PMID 17400026. http://www.sciencedirect.com/science/article/pii/S1521693407000375.
- ↑ "Deaths: Preliminary Data for 2004". National Center for Health Statistics. June 2019. https://www.cdc.gov/nchs/data/hestat/prelimdeaths04/preliminarydeaths04.htm.
- ↑ "Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood". Cardiovascular Research 81 (4): 713–22. March 2009. doi:10.1093/cvr/cvn341. PMID 19088083.
- ↑ "Massive pulmonary haemorrhage: a cause of sudden unexpected deaths in severely growth retarded infants". Australian Paediatric Journal 17 (1): 32–4. March 1981. doi:10.1111/j.1440-1754.1981.tb00010.x. PMID 7247876.
- ↑ "Hyaline Membrane Disease". EMedicine. 27 April 2022. http://emedicine.medscape.com/article/409409-overview.
- ↑ "National Practitioner Data Bank 2006 Annual Report". http://www.npdb-hipdb.hrsa.gov/pubs/stats/2006_NPDB_Annual_Report.pdf.
External links
- Zanelli, Santina A (3 April 2021). "Hypoxic-Ischemic Brain Injury in the Newborn". Medscape (WebMD LLC). http://emedicine.medscape.com/article/1183351-overview.
- Zanelli, Santina A (3 April 2021). "Hypoxic-Ischemic Encephalopathy". Medscape (WebMD LLC). http://emedicine.medscape.com/article/973501-overview.
- Johnson, Kate. "Clear Criteria for Defining Birth Asphyxia". Medscape. WebMD LLC. http://www.medscape.com/viewarticle/452726.
| Classification |
|---|
 |