Medicine:Pretargeting (imaging)
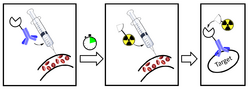
Pretargeting (imaging) is a tool for nuclear medicine and radiotherapy. Imaging studies require a high contrast of target to background. This can be provided by using a biomarker which has a high affinity and specificity for its target (e.g. an antibody).
History
The beginning of antibody imaging
Owing to their high affinity and specificity, antibodies have been considered as suitable vehicles for imaging and therapeutics, since the beginning of the 20th Century.[1][2][3]
The first radiolabelled antibodies were used in the early 1950s and got used for cancer therapy,[4][5] but it took roughly two more decades before it was demonstrated that they target human tumour associated antigens in cancer patients.[6] Due to the hybridoma technology in 1975, monoclonal (murine) antibodies could easily be produced in practical amounts,[7] consequently the number of studies increased drastically. However, these types of antibodies turned out to be quite troublesome, due to the triggering of the human anti-murine antibody response.[8] Consequently chimeric, humanised and human monoclonal antibodies have been created, produced and get used nowadays.[9]
Owing to the high molecular weight of antibodies and the Fc domain of the antibody,[10] a slow clearance from the blood and non-target tissue occurs, which results in low tumour-to-blood and tumour-to-muscle ratios.[11][12] Because of this, antibodies which are going to be used for imaging purposes need to be labelled with radionuclides that have a long half-life,[13] which increases the radiation dose to the patient. This consequently encouraged the development of lower molecular weight antibodies and resulted in the development of minibodies, diabodies, single chain variable fragments (scFv) and single domain fragments (Fv).[14]
Development of pretargeted imaging
To bypass the problem associated with the prolonged circulation time of radiolabelled antibodies, in the mid-1980s a strategy called pretargeted radioimmunotherapy was developed.[15][16] In short, this approach contained two important steps: 1. administration of a macromolecular targeting vector (usually antibody-based), and 2. a small radiolabelled molecule, which interacts with the targeting vector. Most importantly the small radiolabelled molecule gets injected after a predetermined lag period after which the macromolecule has had enough time to bind to its target and the residual unbound macromolecule to be cleared out of the system.[14] To ensure sufficient interaction between the two components, suitable modifications with complementary species are required (like bioorthogonal modifications).[17]
Pretargeting strategies can lead to an improved imaging contrast, as it combines the high target specificity and affinity of an antibody with the fast pharmacokinetic properties of a small molecule. The concept of pretargeting, although existing for several decades already, was limited to a few distinct classes. Developing chemical reactions that proceed quickly within living systems, without interacting with the large variety of existing functional groups, used to be an inherent difficulty. However, there have been several advancements in this area over the past few years.[14]
Conventional pretargeting systems
Bispecific antibodies and radiolabelled haptens
The beginning of the pretargeting concept was based on bispecific antibodies which were able to bind a specific target antigen and a radiolabelled hapten.[18][19][20][21][22][23] Possible was this approach because of the development of monoclonal Antibodies which could be connected to radiometal chelates.[15] Also connecting two haptens via a two amino acid linker resulted in an enhancement effect of the affinity, which improved the uptake and retention of the radiolabelled compound without affecting the rapid clearance.[24]
Limiting factor of this approach were the slow binding constant which was rarely higher than 10−10 M, amongst other reasons.[14]
Biotin-(strept)avidin
After the discovery of the fast interaction between Biotin and (Strept)Avidin, which show a high binding affinity, this approach has been used in many different ways (e.g. for protein purification purposes like the Step-tag).[25][26][27][28][29][30][31]
References
- ↑ Wu, Anna M.; Olafsen, Tove (May 2008). "Antibodies for Molecular Imaging of Cancer". The Cancer Journal 14 (3): 191–197. doi:10.1097/ppo.0b013e31817b07ae. ISSN 1528-9117. PMID 18536559. http://dx.doi.org/10.1097/ppo.0b013e31817b07ae.
- ↑ Knowles, Scott M.; Wu, Anna M. (2012-11-01). "Advances in Immuno–Positron Emission Tomography: Antibodies for Molecular Imaging in Oncology" (in en). Journal of Clinical Oncology 30 (31): 3884–3892. doi:10.1200/JCO.2012.42.4887. ISSN 0732-183X. PMID 22987087.
- ↑ Boerman, Otto C.; Oyen, Wim J. G. (2011-08-01). "Immuno-PET of Cancer: A Revival of Antibody Imaging" (in en). Journal of Nuclear Medicine 52 (8): 1171–1172. doi:10.2967/jnumed.111.089771. ISSN 0161-5505. PMID 21764784. http://jnm.snmjournals.org/content/52/8/1171.
- ↑ Pressman, David; Korngold, Leonhard (1953). "The in vivo localization of anti-Wagner-osteogenic-sarcoma antibodies" (in en). Cancer 6 (3): 619–623. doi:10.1002/1097-0142(195305)6:3<619::aid-cncr2820060319>3.0.co;2-y. ISSN 1097-0142. PMID 13042784.
- ↑ Wisser, R.W (September 1956). "A Study of the Preparation, Localization, and Effects of Antitumor Antibodies Labeled with I131". Cancer Research 16 (8): 761–773. PMID 13364902. https://cancerres.aacrjournals.org/content/16/8/761.short.
- ↑ Goldenberg, David M.; DeLand, Frank; Kim, Euishin; Bennett, Sidney; Primus, F. James; van Nagell, John R.; Estes, Norman; DeSimone, Philip et al. (1978-06-22). "Use of Radio-Labeled Antibodies to Carcinoembryonic Antigen for the Detection and Localization of Diverse Cancers by External Photoscanning" (in en). New England Journal of Medicine 298 (25): 1384–1388. doi:10.1056/NEJM197806222982503. ISSN 0028-4793. PMID 349387. http://www.nejm.org/doi/abs/10.1056/NEJM197806222982503.
- ↑ Köhler, G.; Milstein, C. (August 1975). "Continuous cultures of fused cells secreting antibody of predefined specificity" (in en). Nature 256 (5517): 495–497. doi:10.1038/256495a0. ISSN 1476-4687. PMID 1172191. Bibcode: 1975Natur.256..495K. https://www.nature.com/articles/256495a0.
- ↑ Tjandra, J. J.; Ramadi, L.; McKenzie, I. F. (December 1990). "Development of human anti-murine antibody (HAMA) response in patients". Immunology and Cell Biology 68 ( Pt 6) (6): 367–376. doi:10.1038/icb.1990.50. ISSN 0818-9641. PMID 1711007. https://www.ncbi.nlm.nih.gov/pubmed/1711007.
- ↑ Chames, Patrick; Van Regenmortel, Marc; Weiss, Etienne; Baty, Daniel (May 2009). "Therapeutic antibodies: successes, limitations and hopes for the future". British Journal of Pharmacology 157 (2): 220–233. doi:10.1111/j.1476-5381.2009.00190.x. ISSN 0007-1188. PMID 19459844.
- ↑ Lee, Chang-Han; Kang, Tae Hyun; Godon, Ophélie; Watanabe, Makiko; Delidakis, George; Gillis, Caitlin M.; Sterlin, Delphine; Hardy, David et al. (December 2019). "An engineered human Fc domain that behaves like a pH-toggle switch for ultra-long circulation persistence" (in en). Nature Communications 10 (1): 5031. doi:10.1038/s41467-019-13108-2. ISSN 2041-1723. PMID 31695028. Bibcode: 2019NatCo..10.5031L.
- ↑ Rosebrough, S. F.; Grossman, Z. D.; McAfee, J. G.; Kudryk, B. J.; Subramanian, G.; Ritter-Hrncirik, C. A.; Witanowski, L. S.; Tillapaugh-Fay, G. et al. (July 1988). "Thrombus imaging with indium-111 and iodine-131-labeled fibrin-specific monoclonal antibody and its F(ab')2 and Fab fragments". Journal of Nuclear Medicine 29 (7): 1212–1222. ISSN 0161-5505. PMID 3392581. https://www.ncbi.nlm.nih.gov/pubmed/3392581.
- ↑ Murray, J. L.; Rosenblum, M. G.; Lamki, L.; Glenn, H. J.; Krizan, Z.; Hersh, E. M.; Plager, C. E.; Bartholomew, R. M. et al. (January 1987). "Clinical parameters related to optimal tumor localization of indium-111-labeled mouse antimelanoma monoclonal antibody ZME-018". Journal of Nuclear Medicine 28 (1): 25–33. ISSN 0161-5505. PMID 3794809. https://www.ncbi.nlm.nih.gov/pubmed/3794809.
- ↑ Deri, Melissa A.; Zeglis, Brian M.; Francesconi, Lynn C.; Lewis, Jason S. (January 2013). "PET Imaging with 89Zr: From Radiochemistry to the Clinic". Nuclear Medicine and Biology 40 (1): 3–14. doi:10.1016/j.nucmedbio.2012.08.004. ISSN 0969-8051. PMID 22998840.
- ↑ 14.0 14.1 14.2 14.3 Knight, James C; Cornelissen, Bart (2014-03-20). "Bioorthogonal chemistry: implications for pretargeted nuclear (PET/SPECT) imaging and therapy". American Journal of Nuclear Medicine and Molecular Imaging 4 (2): 96–113. ISSN 2160-8407. PMID 24753979.
- ↑ 15.0 15.1 Reardan, Dayton T.; Meares, Claude F.; Goodwin, David A.; McTigue, Maureen; David, Gary S.; Stone, Mary R.; Leung, Julia P.; Bartholomew, Richard M. et al. (July 1985). "Antibodies against metal chelates" (in en). Nature 316 (6025): 265–268. doi:10.1038/316265a0. ISSN 0028-0836. PMID 3927170. Bibcode: 1985Natur.316..265R. http://www.nature.com/articles/316265a0.
- ↑ Goodwin, D. A.; Mears, C. F.; McTigue, M.; David, G. S. (August 1986). "Monoclonal antibody hapten radiopharmaceutical delivery". Nuclear Medicine Communications 7 (8): 569–580. doi:10.1097/00006231-198608000-00002. ISSN 0143-3636. PMID 3095721. https://www.ncbi.nlm.nih.gov/pubmed/3095721.
- ↑ Goodwin, D. A.; Meares, C. F.; McCall, M. J.; McTigue, M.; Chaovapong, W. (February 1988). "Pre-targeted immunoscintigraphy of murine tumors with indium-111-labeled bifunctional haptens". Journal of Nuclear Medicine 29 (2): 226–234. ISSN 0161-5505. PMID 3346734. https://www.ncbi.nlm.nih.gov/pubmed/3346734.
- ↑ Sharkey, Robert M.; Rossi, Edmund A.; Chang, Chien-Hsing; Goldenberg, David M. (February 2010). "Improved Cancer Therapy and Molecular Imaging with Multivalent, Multispecific Antibodies" (in en). Cancer Biotherapy and Radiopharmaceuticals 25 (1): 1–12. doi:10.1089/cbr.2009.0690. ISSN 1084-9785. PMID 20187791.
- ↑ Goldenberg, D. M.; Rossi, E. A.; Sharkey, R. M.; McBride, W. J.; Chang, C.-H. (2007-12-12). "Multifunctional Antibodies by the Dock-and-Lock Method for Improved Cancer Imaging and Therapy by Pretargeting" (in en). Journal of Nuclear Medicine 49 (1): 158–163. doi:10.2967/jnumed.107.046185. ISSN 0161-5505. PMID 18077530.
- ↑ Goldenberg, David M.; Chatal, Jean-Francois; Barbet, Jacques; Boerman, Otto; Sharkey, Robert M. (March 2007). "Cancer imaging and therapy with bispecific antibody pretargeting" (in en). Update on Cancer Therapeutics 2 (1): 19–31. doi:10.1016/j.uct.2007.04.003. PMID 18311322.
- ↑ Sharkey, Robert M.; Karacay, Habibe; Goldenberg, David M. (2010). "Improving the treatment of non-Hodgkin lymphoma with antibody-targeted radionuclides" (in en). Cancer 116 (S4): 1134–1145. doi:10.1002/cncr.24802. ISSN 1097-0142. PMID 20127947.
- ↑ Sharkey, Robert M.; Rossi, Edmund A.; McBride, William J.; Chang, Chien-Hsing; Goldenberg, David M. (May 2010). "Recombinant Bispecific Monoclonal Antibodies Prepared by the Dock-and-Lock Strategy for Pretargeted Radioimmunotherapy" (in en). Seminars in Nuclear Medicine 40 (3): 190–203. doi:10.1053/j.semnuclmed.2009.12.002. PMID 20350628.
- ↑ Goldenberg, David M.; Chang, Chien-Hsing; Rossi, Edmund A.; J, William; McBride, null; Sharkey, Robert M. (2012). "Pretargeted molecular imaging and radioimmunotherapy". Theranostics 2 (5): 523–540. doi:10.7150/thno.3582. ISSN 1838-7640. PMID 22737190.
- ↑ Le Doussal, J. M.; Martin, M.; Gautherot, E.; Delaage, M.; Barbet, J. (August 1989). "In vitro and in vivo targeting of radiolabeled monovalent and divalent haptens with dual specificity monoclonal antibody conjugates: enhanced divalent hapten affinity for cell-bound antibody conjugate". Journal of Nuclear Medicine 30 (8): 1358–1366. ISSN 0161-5505. PMID 2787847. https://www.ncbi.nlm.nih.gov/pubmed/2787847.
- ↑ Hnatowich, D. J.; Virzi, F.; Rusckowski, M. (August 1987). "Investigations of avidin and biotin for imaging applications". Journal of Nuclear Medicine 28 (8): 1294–1302. ISSN 0161-5505. PMID 3612292. https://www.ncbi.nlm.nih.gov/pubmed/3612292.
- ↑ Michael Green, N. (1990), "[5 Avidin and streptavidin"] (in en), Avidin-Biotin Technology, Methods in Enzymology, 184, Elsevier, pp. 51–67, doi:10.1016/0076-6879(90)84259-j, ISBN 978-0-12-182085-5, PMID 2388586, https://linkinghub.elsevier.com/retrieve/pii/007668799084259J, retrieved 2020-09-30
- ↑ Boerman, Otto C.; van Schaijk, Frank G.; Oyen, Wim J. G.; Corstens, Frans H. M. (March 2003). "Pretargeted radioimmunotherapy of cancer: progress step by step". Journal of Nuclear Medicine 44 (3): 400–411. ISSN 0161-5505. PMID 12621007. https://www.ncbi.nlm.nih.gov/pubmed/12621007.
- ↑ Liu, Guozheng; Hnatowich, Donald J. (2008-11-19). "A Semiempirical Model of Tumor Pretargeting". Bioconjugate Chemistry 19 (11): 2095–2104. doi:10.1021/bc8002748. ISSN 1043-1802. PMID 18839978.
- ↑ Sharkey, Robert M.; Chang, Chien-Hsing; Rossi, Edmund A.; McBride, William J.; Goldenberg, David M. (June 2012). "Pretargeting: taking an alternate route for localizing radionuclides" (in en). Tumor Biology 33 (3): 591–600. doi:10.1007/s13277-012-0367-6. ISSN 1010-4283. PMID 22396041. http://link.springer.com/10.1007/s13277-012-0367-6.
- ↑ Sharkey, Robert M.; Goldenberg, David M. (2006-01-01). "Advances in Radioimmunotherapy in the Age of Molecular Engineering and Pretargeting". Cancer Investigation 24 (1): 82–97. doi:10.1080/07357900500449553. ISSN 0735-7907. PMID 16466997. https://doi.org/10.1080/07357900500449553.
- ↑ Goldenberg, David M.; Sharkey, Robert M.; Paganelli, Giovanni; Barbet, Jacques; Chatal, Jean-François (2006-02-10). "Antibody Pretargeting Advances Cancer Radioimmunodetection and Radioimmunotherapy". Journal of Clinical Oncology 24 (5): 823–834. doi:10.1200/jco.2005.03.8471. ISSN 0732-183X. PMID 16380412. http://dx.doi.org/10.1200/jco.2005.03.8471.
 |