Medicine:Radioimmunotherapy
| Radioimmunotherapy | |
|---|---|
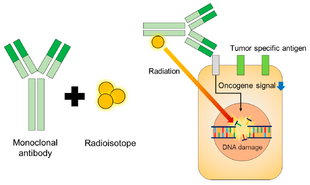 Schematic of radioimmunotherapy (RIT) | |
| Other names | RIT |
| ICD-9-CM | 92.28 |
| MeSH | D016499 |
Radioimmunotherapy (RIT) uses an antibody labeled with a radionuclide to deliver cytotoxic radiation to a target cell.[1] It is a form of unsealed source radiotherapy. In cancer therapy, an antibody with specificity for a tumor-associated antigen is used to deliver a lethal dose of radiation to the tumor cells. The ability for the antibody to specifically bind to a tumor-associated antigen increases the dose delivered to the tumor cells while decreasing the dose to normal tissues. By its nature, RIT requires a tumor cell to express an antigen that is unique to the neoplasm or is not accessible in normal cells.
History of available agents
| Name | Description | FDA status | EMA status |
| Ibritumomab tiuxetan (Zevalin) | monoclonal antibody anti-CD20 conjugated to a molecule that chelates Yttrium-90. | Approved (2002)[2][3] | Authorised (2004)[4] |
| Iodine (131I) tositumomab (Bexxar) | links a molecule containing Iodine-131 to an anti-CD20 monoclonal antibody | Approved (2003)[5] Withdrawn (2014)[6] |
Orphan drug (2003) Withdrawn (2015)[7] |
| Lutetium (177Lu) lilotomab satetraxetan (Betalutin) | combination of lutetium-177 and an anti-CD37 monoclonal antibody | Fast track (2020)[8] | Orphan drug (2020)[9] |
131I tositumomab and 90Y ibritumomab tiuxetan were the first agents of radioimmunotherapy, and they were approved for the treatment of refractory non-Hodgkin's lymphoma. This means they are used in patients whose lymphoma is refractory to conventional chemotherapy and the monoclonal antibody rituximab.
Agents in clinical development
A set of radioimmunotherapy drugs that rely upon an alpha-emitting isotope (e.g., bismuth-213 or, preferably, actinium-225), rather than a beta emitter, as the killing source of radiation is being developed. Several phase II clinical trials for the treatment of acute myeloid leukemia have been carried out using alpha-emitting RITs.[10][11]
90Y-FF-21101 is a monoclonal antibody against P-cadherin radiolabeled with yttrium-90.[12] It is one of several RIT treatments under investigation intending to treat solid tumors.[13] A phase I clinical trial began in 2015.[14]
Other applications (non-approved indications)
Other types of cancer for which RIT has therapeutic potential include prostate cancer,[15] metastatic melanoma,[16] ovarian cancer,[17] neoplastic meningitis,[17] leukemia,[18] high-grade brain glioma,[19] and metastatic colorectal cancer.[20]
Components of the extracellular matrix and the tumor microenvironment can also be targeted by radioimmunotherapy, such as Netrin-1 [21] (an axon guidance protein) and FAP (a marker for cancer associated fibroblasts).[22]
References
- ↑ Milenic, Diane E.; Brady, Erik D.; Brechbiel, Martin W. (June 2004). "Antibody-targeted radiation cancer therapy". Nature Reviews Drug Discovery 3 (6): 488–499. doi:10.1038/nrd1413. PMID 15173838. https://zenodo.org/record/1233515.
- ↑ FIbritumomab Tiuxetan (Zevalin™) Radioimmunotherapy of Non-Hodgkin’s Lymphoma
- ↑ Rao AV, Akabani G, Rizzieri DA. Radioimmunotherapy for Non-Hodgkin's Lymphoma. Clin Med Res. 2005 Aug;3(3):157-65.
- ↑ "Zevalin". https://www.ema.europa.eu/en/medicines/human/EPAR/zevalin. Retrieved 8 November 2020.
- ↑ Tositumomab and Iodine I 131 Tositumomab – Product Approval Information – Licensing Action
- ↑ "Why Good Drugs Sometimes Fail: The Bexxar Story". 2013-08-26. http://www.xconomy.com/national/2013/08/26/why-good-drugs-sometimes-fail-in-the-market-the-bexxar-story/.
- ↑ "EU/3/03/136". https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu303136. Retrieved 8 November 2020.
- ↑ "FDA grants fast track status to Betalutin for marginal zone lymphoma". Healio. 29 June 2020. https://www.healio.com/news/hematology-oncology/20200629/fda-grants-fast-track-status-to-betalutin-for-marginal-zone-lymphoma.
- ↑ "EU/3/20/2280". https://www.ema.europa.eu/en/medicines/human/orphan-designations/eu3202280. Retrieved 8 November 2020.
- ↑ Bodet-Milin, Caroline; Kraeber-Bodéré, Françoise; Eugène, Thomas; Guérard, François; Gaschet, Joëlle; Bailly, Clément; Mougin, Marie; Bourgeois, Mickaël et al. (March 2016). "Radioimmunotherapy for Treatment of Acute Leukemia". Seminars in Nuclear Medicine 46 (2): 135–146. doi:10.1053/j.semnuclmed.2015.10.007. PMID 26897718.
- ↑ Pandit-Taskar, Neeta (December 2019). "Targeted Radioimmunotherapy and Theranostics with Alpha Emitters". Journal of Medical Imaging and Radiation Sciences 50 (4): S41–S44. doi:10.1016/j.jmir.2019.07.006. PMID 31451417. https://www.jmirs.org/article/S1939-8654(19)30348-0/fulltext.
- ↑ Subbiah, Vivek; Erwin, William; Mawlawi, Osama; McCoy, Asa; Wages, David; Wheeler, Catherine; Gonzalez-Lepera, Carlos; Liu, Holly et al. (18 August 2020). "Phase I Study of P-cadherin–targeted Radioimmunotherapy with 90 Y-FF-21101 Monoclonal Antibody in Solid Tumors". Clinical Cancer Research 26 (22): 1078–0432.CCR–20-0037. doi:10.1158/1078-0432.CCR-20-0037. PMID 32816889.
- ↑ Zaheer, Javeria; Kim, Hyeongi; Lee, Yong-Jin; Kim, Jin Su; Lim, Sang Moo (8 November 2019). "Combination Radioimmunotherapy Strategies for Solid Tumors". International Journal of Molecular Sciences 20 (22): 5579. doi:10.3390/ijms20225579. PMID 31717302.
- ↑ A Phase 1 Dose-escalation Study of Radio- Labeled Antibody, FF-21101(90Y) for the Treatment of Advanced Cancer
- ↑ Smith-Jones PM. Radioimmunotherapy of prostate cancer. Q J Nucl Med Mol Imaging. 2004 Dec;48(4):297-304.
- ↑ Dadachova E, Nosanchuk JD, Shi L, Schweitzer AD, Frenkel A, Nosanchuk JS, and Casadevall A. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a monoclonal antibody to melanin. Proc Natl Acad Sci USA 2004;101: 14865-70.
- ↑ 17.0 17.1 Zalutsky MR, Pozzi OR. Radioimmunotherapy with alpha-particle emitting radionuclides. Q J Nucl Med Mol Imaging. 2004 Dec;48(4):289-96.
- ↑ Burke JM, Jurcic JG. Radioimmunotherapy of leukemia. Adv Pharmacol. 2004;51:185-208.
- ↑ Quang TS, Brady LW. Radioimmunotherapy as a novel treatment regimen: 125I-labeled monoclonal antibody 425 in the treatment of high-grade brain gliomas. Int J Radiat Oncol Biol Phys. 2004 Mar 1;58(3):972-5.
- ↑ Wong JY, Shibata S, Williams LE, Kwok CS, Liu A, Chu DZ, Yamauchi DM, Wilczynski S, Ikle DN, Wu AM, Yazaki PJ, Shively JE, Doroshow JH, Raubitschek AA. A Phase I trial of 90Y-anti-carcinoembryonic antigen chimeric T84.66 radioimmunotherapy with 5-fluorouracil in patients with metastatic colorectal cancer. Clin Cancer Res. 2003 Dec 1;9(16 Pt 1):5842-52
- ↑ Kryza D, Wischhusen J, Richaud M, Hervieu M, Sidi Boumedine J, Delcros JG, Besse S, Baudier T, Laval PA, Breusa S, Boutault E, Clermidy H, Rama N, Ducarouge B, Devouassoux-Shisheboran M, Chezal JM, Giraudet AL, Walter T, Mehlen P, Sarrut D, Gibert B.From netrin-1-targeted SPECT/CT to internal radiotherapy for management of advanced solid tumors. EMBO Mol Med. 2023 Apr 11;15(4):e16732. doi: 10.15252/emmm.202216732. Epub 2023 Mar 6. PMID: 36876343
- ↑ Marko Magdi Abdou Sidrak, Maria Silvia De Feo, Ferdinando Corica, Joana Gorica, Miriam Conte, Luca Filippi, Orazio Schillaci, Giuseppe De Vincentis, and Viviana Frantellizzi1,Fibroblast Activation Protein Inhibitor (FAPI)-Based Theranostics—Where We Are at and Where We Are Heading: A Systematic Review Int J Mol Sci. 2023 Feb; 24(4): 3863. Published online 2023 Feb 15. doi: 10.3390/ijms24043863
External links
- Radioimmunotherapy at the US National Library of Medicine Medical Subject Headings (MeSH)
- Radioimmunotherapy.org
 |

