Medicine:Reticular dysgenesis
Reticular dysgenesis (RD) is a rare, inherited autosomal recessive disease that results in immunodeficiency.[1] Individuals with RD have mutations in both copies of the AK2 gene.[1] Mutations in this gene lead to absence of AK2 protein.[2] AK2 protein allows hematopoietic stem cells to differentiate and proliferate.[2] Hematopoietic stem cells give rise to blood cells.[2]
Differentiation and proliferation of hematopoietic stem cells require a lot of energy and this energy is supplied by the mitochondria.[2] The energy metabolism of mitochondria is regulated by the AK2 protein.[2] If there is a mutation in the protein, that means that the mitochondria metabolism most likely will be altered and will not be able to provide enough energy to the hematopoietic stem cells.[2] As a result, hematopoietic stem cells will not be able to differentiate or proliferate.[2]
The immune system consists of specialized cells that work together to fight off bacteria, fungi and viruses.[3] These cells include T lymphocytes (T cells), that primarily mediate the immune system, B lymphocytes (B cells) and Natural Killer cells.[3] Patients with RD have a genetic defect that affects the T cells and at least one other type of immune cell.[4] Since more than one type of immune cell is affected, this disease is classified as a severe combined immunodeficiency disease (SCID).[3] A weakened immune system leaves patients susceptible to different kinds of infection. Commonly, patients who are diagnosed with RD also have bacterial sepsis and/or pneumonia.[4] The annual incidence has been estimated at 1/3,000,000-1/5,000,000 and both females and males are affected.[5]
Signs and symptoms
| Signs and Symptoms | Approximate Number of Patients Affected |
|---|---|
| Abnormality of mitochondria metabolism | 90% |
| Abnormality of Neutrophils | 90% |
| Anemia | 90% |
| Aplasia/Hypoplasia of the thymus | 90% |
| Cellular immunodeficiency | 90% |
| Decreased antibody level in blood | 90% |
| Diarrhea | 90% |
| Hearing Impairment | 90% |
| Recurrent respiratory infection | 90% |
| Sepsis | 90% |
| Abnormality of temperature regulation | 50% |
| Malabsorption | 50% |
| Weight Loss | 50% |
| Dehydration | 7.5% |
| Skin rash | 7.5% |
| Skin Ulcer | 7.5% |
Risk factors
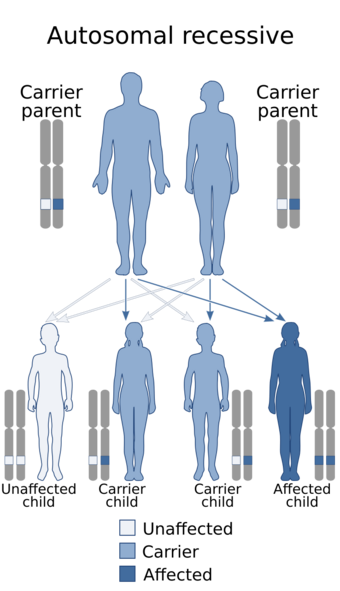
- Condition follows an autosomal recessive pattern[7]
Diagnosis
Health professionals must look at a person's history, symptoms, physical exam and laboratory test in order to make a diagnosis. If the results show patients with low levels of lymphocytes, absence of granulocytes or absence of thymus then the patient may be suspected to have RD.[4]
Treatment
RD can only be treated temporarily through hematopoietic stem cell transplantation (HSCT) and cytokine therapy.[4][8][9]
Hematopoietic stem cell transplantation
Transplantation of stem cells are taken from the bone marrow, peripheral blood or umbilical cord of healthy, matched donors.[10] Hematopoietic stem cell transplantation (HSCT) involves intravenous infusion of stem cells to those who have either a damaged bone marrow or defective immune system.[4][10] Transplantation is a simple process. Bone marrow product is infused through a central vein over a period of several hours.[10] The hematopoietic cells are able to go to the bone marrow through tracking mechanisms.[10] Patients who suffer from RD will now have more stem cells that can differentiate into immune cells.[citation needed]
Cytokine Therapy
Recombinant granulocyte-macrophage colony-stimulating factor (rGM-CSF) can be used as a temporary cure.[8] GM-CSF stimulates production of white blood cells.[8] This cure is commonly used in patients who are awaiting bone marrow transplantation.[8] Response to this cure can vary.[8] Those with a more severe combined immunodeficiency may have no response to this therapy.[8]
Prognosis
The survival range is estimated to be 3 days to 17 weeks without treatment.[8] Patients die due to bacterial or viral infections.[8] Aggressive treatment with antibiotics is required and bone marrow transplant is common.[8] Patients undergoing bone marrow transplant, specifically from a matched sibling, have a higher 5 year survival rate than those receiving a transplant from other donors.[10]
Research
Gene Therapy
Gene therapy is a relatively new concept in the field of SCID.[11] This therapy is currently undergoing clinical trial and has cured a small number of children suffering from X-linked SCID and recessive allele SCID.[11] Gene therapy aims to correct the underlying genetic abnormality in SCID.[11] In the case of RD, the genetic abnormality would be AK2 malfunction.[2] Stem cells are taken from an affected child's blood or bone marrow.[11] Then in laboratory conditions the stem cells are manipulated and corrected with gene technology.[11] They are then injected back into the patient.[11] Similarly, in bone transplant, stem cells are able to find their way back through tracking mechanisms.[10][11]
References
- ↑ 1.0 1.1 Pannicke, Ulrich; Hönig, Manfred; Hess, Isabell; Friesen, Claudia; Holzmann, Karlheinz; Rump, Eva-Maria; Barth, Thomas F; Rojewski, Markus T et al. (2009). "Reticular dysgenesis (aleukocytosis) is caused by mutations in the gene encoding mitochondrial adenylate kinase 2". Nature Genetics 41 (1): 101–105. doi:10.1038/ng.265. PMID 19043417.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Six, E.; Lagresle-Peyrou, C.; Susini, S.; De Chappedelaine, C.; Sigrist, N.; Sadek, H.; Chouteau, M.; Cagnard, N. et al. (2015-08-13). "AK2 deficiency compromises the mitochondrial energy metabolism required for differentiation of human neutrophil and lymphoid lineages" (in en). Cell Death & Disease 6 (8): e1856. doi:10.1038/cddis.2015.211. PMID 26270350.
- ↑ 3.0 3.1 3.2 "The Immune System and Primary Immunodeficiency | Immune Deficiency Foundation". http://primaryimmune.org/about-primary-immunodeficiencies/relevant-info/the-immune-system/.
- ↑ 4.0 4.1 4.2 4.3 4.4 Bertrand, Y.; Müller, S. M.; Casanova, J. L.; Morgan, G.; Fischer, A.; Friedrich, W. (2002-05-01). "Reticular dysgenesis: HLA non-identical bone marrow transplants in a series of 10 patients". Bone Marrow Transplantation 29 (9): 759–762. doi:10.1038/sj.bmt.1703531. ISSN 0268-3369. PMID 12040473.
- ↑ "Reticular dysgenesis: epidemiology". Genetic and rare diseases information center. https://rarediseases.info.nih.gov/diseases/8625/reticular-dysgenesis.
- ↑ "Reticular dysgenesis". https://rarediseases.info.nih.gov/diseases/8625/reticular-dysgenesis.
- ↑ 7.0 7.1 7.2 "Severe Combined Immunodeficiency - NORD (National Organization for Rare Disorders)" (in en-US). NORD (National Organization for Rare Disorders). https://rarediseases.org/rare-diseases/severe-combined-immunodeficiency/.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 Calhoun, Christensen, DA, RD (1998). "Recent advances in the pathogenesis and treatment of nonimmune neutropenias in the neonate". Current Opinion in Hematology 5 (1): 37–41. doi:10.1097/00062752-199801000-00007. PMID 9515201.
- ↑ Scheinfeld, Noah (2019-08-05). Intravenous Immunoglobulin. Medscape. http://emedicine.medscape.com/article/210367-overview?pa=PmDve%2FalcQVFwj6gs9oO35bXOkN3F7qRBlYh4a9jWMjretDc%2F9eVnMnljEwh2N9BCireY%2BwZp%2FVchUGrFPrk1CchrzF%2F7vlnSF6AEX%2F09M8%3D.
- ↑ 10.0 10.1 10.2 10.3 10.4 10.5 Perumbeti, Ajay (2018-08-06). Hematopoietic Stem Cell Transplantation. http://emedicine.medscape.com/article/208954-overview.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 "Severe combined immunodeficiency (SCID)". http://www.gosh.nhs.uk/medical-information-0/search-medical-conditions/severe-combined-immunodeficiency-scid.
External links
| Classification | |
|---|---|
| External resources |
 |