Biology:Natural killer cell
| Natural killer cell | |
|---|---|
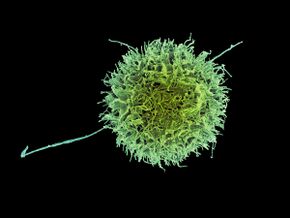 Human natural killer cell, colorized scanning electron micrograph | |
| Details | |
| System | Immune system |
| Function | Cytotoxic lymphocyte |
| Anatomical terms of microanatomy | |
Natural killer cells, also known as NK cells or large granular lymphocytes (LGL), are a type of cytotoxic lymphocyte critical to the innate immune system. They belong to the rapidly expanding family of known innate lymphoid cells (ILC) and represent 5–20% of all circulating lymphocytes in humans.[1] The role of NK cells is analogous to that of cytotoxic T cells in the vertebrate adaptive immune response. NK cells provide rapid responses to virus-infected cell and other intracellular pathogens acting at around 3 days after infection, and respond to tumor formation. Most immune cells detect the antigen presented on major histocompatibility complex (MHC) on infected cell surfaces, but NK cells can recognize and kill stressed cells in the absence of antibodies and MHC, allowing for a much faster immune reaction. They were named "natural killers" because of the notion that they do not require activation to kill cells that are missing "self" markers of MHC class I.[2] This role is especially important because harmful cells that are missing MHC I markers cannot be detected and destroyed by other immune cells, such as T lymphocyte cells.
NK cells can be identified by the presence of CD56 and the absence of CD3 (CD56+, CD3−).[3] NK cells differentiate from CD127+ common innate lymphoid progenitor,[4] which is downstream of the common lymphoid progenitor from which B and T lymphocytes are also derived.[4][5] NK cells are known to differentiate and mature in the bone marrow, lymph nodes, spleen, tonsils, and thymus, where they then enter into the circulation.[6] NK cells differ from natural killer T cells (NKTs) phenotypically, by origin and by respective effector functions; often, NKT cell activity promotes NK cell activity by secreting interferon gamma. In contrast to NKT cells, NK cells do not express T-cell antigen receptors (TCR) or pan T marker CD3 or surface immunoglobulins (Ig) B cell receptors, but they usually express the surface markers CD16 (FcγRIII) and CD57 in humans, NK1.1 or NK1.2 in C57BL/6 mice. The NKp46 cell surface marker constitutes, at the moment, another NK cell marker of preference being expressed in both humans, several strains of mice (including BALB/c mice) and in three common monkey species.[7][8]
Outside of innate immunity, both activating and inhibitory NK cell receptors play important functional roles in self tolerance and the sustaining of NK cell activity. NK cells also play a role in the adaptive immune response:[9] numerous experiments have demonstrated their ability to readily adjust to the immediate environment and formulate antigen-specific immunological memory, fundamental for responding to secondary infections with the same antigen.[10] The role of NK cells in both the innate and adaptive immune responses is becoming increasingly important in research using NK cell activity as a potential cancer therapy and HIV therapy.[11][12]
Early history
In early experiments on cell-mediated cytotoxicity against tumor target cells, both in cancer patients and animal models, investigators consistently observed what was termed a "natural" reactivity; that is, a certain population of cells seemed to be able to destroy tumor cells without having been previously sensitized to them. The first published study to assert that untreated lymphoid cells were able to confer a natural immunity to tumors was performed by Dr. Henry Smith at the University of Leeds School of Medicine in 1966,[13] leading to the conclusion that the "phenomenon appear[ed] to be an expression of defense mechanisms to tumor growth present in normal mice." Other researchers had also made similar observations, but as these discoveries were inconsistent with the established model at the time, many initially considered these observations to be artifacts.[14]
By 1973, 'natural killing' activity was established across a wide variety of species, and the existence of a separate lineage of cells possessing this ability was postulated. The discovery that a unique type of lymphocyte was responsible for "natural" or spontaneous cytotoxicity was made in the early 1970s by doctoral student Rolf Kiessling and postdoctoral fellow Hugh Pross, in the mouse,[15] and by Hugh Pross and doctoral student Mikael Jondal in the human.[16][17] The mouse and human work was carried out under the supervision of professors Eva Klein and Hans Wigzell, respectively, of the Karolinska Institute, Stockholm. Kiessling's research involved the well-characterized ability of T lymphocytes to attack tumor cells which they had been previously immunized against. Pross and Jondal were studying cell-mediated cytotoxicity in normal human blood and the effect of the removal of various receptor-bearing cells on this cytotoxicity. Later that same year, Ronald Herberman published similar data with respect to the unique nature of the mouse effector cell.[18] The human data were confirmed, for the most part, by West et al.[19] using similar techniques and the same erythroleukemic target cell line, K562. K562 is highly sensitive to lysis by human NK cells and, over the decades, the K562 51chromium-release assay has become the most commonly used assay to detect human NK functional activity.[20] Its almost universal use has meant that experimental data can be compared easily by different laboratories around the world.
Using discontinuous density centrifugation, and later monoclonal antibodies, natural killing ability was mapped to the subset of large, granular lymphocytes known today as NK cells. The demonstration that density gradient-isolated large granular lymphocytes were responsible for human NK activity, made by Timonen and Saksela in 1980,[21] was the first time that NK cells had been visualized microscopically, and was a major breakthrough in the field.
Types
NK cells can be classified as CD56bright or CD56dim.[22][23][3] CD56bright NK cells are similar to T helper cells in exerting their influence by releasing cytokines.[23] CD56bright NK cells constitute the majority of NK cells, being found in bone marrow, secondary lymphoid tissue, liver, and skin.[3] CD56bright NK cells are characterized by their preferential killing of highly proliferative cells,[24] and thus might have an immunoregulatory role. CD56dim NK cells are primarily found in the peripheral blood,[3] and are characterized by their cell killing ability.[23] CD56dim NK cells are always CD16 positive (CD16 is the key mediator of antibody-dependent cellular cytotoxicity, or ADCC).[23] CD56bright can transition into CD56dim by acquiring CD16.[3]
NK cells can eliminate virus-infected cells via CD16-mediated ADCC.[25] All coronavirus disease 2019 (COVID-19) patients show depleted CD56bright NK cells, but CD56dim is only depleted in patients with severe COVID-19.[25]
Receptors
NK cell receptors can also be differentiated based on function. Natural cytotoxicity receptors directly induce apoptosis (cell death) after binding to Fas ligand that directly indicate infection of a cell. The MHC-independent receptors (described above) use an alternate pathway to induce apoptosis in infected cells. Natural killer cell activation is determined by the balance of inhibitory and activating receptor stimulation. For example, if the inhibitory receptor signaling is more prominent, then NK cell activity will be inhibited; similarly, if the activating signal is dominant, then NK cell activation will result.[26]
NK cell receptor types (with inhibitory, as well as some activating members) are differentiated by structure, with a few examples to follow:

Activating receptors
- Ly49 (homodimers), relatively ancient, C-type lectin family receptors, are of multigenic presence in mice, while humans have only one pseudogenic Ly49, the receptor for classical (polymorphic) MHC I molecules.
- NCR (natural cytotoxicity receptors), type 1 transmembrane proteins of the immunoglobulin superfamily, upon stimulation mediate NK killing and release of IFNγ. They bind viral ligands such as hemagglutinins and hemagglutinin neuraminidases, some bacterial ligands and cellular ligands related to tumour growth such as PCNA.
- CD16 (FcγIIIA) plays a role in antibody-dependent cell-mediated cytotoxicity; in particular, they bind immunoglobulin G.
- TLR – Toll-like receptors are receptors that belong in the group of pattern recognition receptors (PRR) which are typical for the cells of innate immunity but are expressed also on NK cells. They recognize PAMPs (pathogen-associated molecular patterns) and DAMPs (damage-associated molecular patterns) as their ligands. These receptors are crucial for the induction of the immune response. TLR induction amplifies the immune response by promoting the production of inflammatory cytokines and chemokines and ultimately leads to the activation of NK cell effector functions.[27] So NK cells directly reacts to the presence of pathogens in its surroundings. Apart from TLR-10 NK cells express all of the human TLR although in various levels. NK cells express high levels of TLR-1, moderate levels of TLR-2, TLR-3, TLR-5 and TLR-6, low levels of TLR-4, TLR-8 and TLR-9 and very low levels of TLR-7.[28] TLR receptors are constitutionally expressed independently of their state of activation and they cooperate with cytokines and chemokines on the activation of the natural killer cells.[29] These receptors are expressed extracellularly on the cell surface or endosomally inside the endosomes. Apart from TLR-3 and TLR-4, all TLR signal through adaptor protein MyD88 which ultimately leads mainly to the activation of NF-κB. TLR-3 signals through the adaptor protein TRIF and TLR-4 can switch between signaling through MyD88 and TRIF respectively. Induction of different TLR leads to distinct activation of NK cell functions.[30]
Inhibitory receptors
- Killer-cell immunoglobulin-like receptors (KIRs) belong to a multigene family of more recently evolved Ig-like extracellular domain receptors; they are present in nonhuman primates, and are the main receptors for both classical MHC I (HLA-A, HLA-B, HLA-C) and nonclassical Mamu-G (HLA-G) in primates. Some KIRs are specific for certain HLA subtypes. Most KIRs are inhibitory and dominant. Regular cells express MHC class 1, so are recognised by KIR receptors and NK cell killing is inhibited.[6]
- CD94/NKG2 (heterodimers), a C-type lectin family receptor, is conserved in both rodents and primates and identifies nonclassical (also nonpolymorphic) MHC I molecules such as HLA-E. Expression of HLA-E at the cell surface is dependent on the presence of nonamer peptide epitope derived from the signal sequence of classical MHC class I molecules, which is generated by the sequential action of signal peptide peptidase and the proteasome. Though indirect, this is a way to survey the levels of classical (polymorphic) HLA molecules.
- ILT or LIR (immunoglobulin-like receptor) – are recently discovered members of the Ig receptor family.
- Ly49 (homodimers) have both activating and inhibitory isoforms. They are highly polymorphic on the population level; though they are structurally unrelated to KIRs, they are the functional homologues of KIRs in mice, including the expression pattern. Ly49s are receptor for classical (polymorphic) MHC I molecules.
Function
Cytolytic granule mediated cell apoptosis
NK cells are cytotoxic; small granules in their cytoplasm contain proteins such as perforin and proteases known as granzymes. Upon release in close proximity to a cell slated for killing, perforin forms pores in the cell membrane of the target cell, creating an aqueous channel through which the granzymes and associated molecules can enter, inducing either apoptosis or osmotic cell lysis. The distinction between apoptosis and cell lysis is important in immunology: lysing a virus-infected cell could potentially release the virions, whereas apoptosis leads to destruction of the virus inside. α-defensins, antimicrobial molecules, are also secreted by NK cells, and directly kill bacteria by disrupting their cell walls in a manner analogous to that of neutrophils.[6]
Antibody-dependent cell-mediated cytotoxicity (ADCC)
Infected cells are routinely opsonized with antibodies for detection by immune cells. Antibodies that bind to antigens can be recognised by FcγRIII (CD16) receptors expressed on NK cells, resulting in NK activation, release of cytolytic granules and consequent cell apoptosis. This is a major killing mechanism of some monoclonal antibodies like rituximab (Rituxan), ofatumumab (Azzera), and others. The contribution of antibody-dependent cell-mediated cytotoxicity to tumor cell killing can be measured with a specific test that uses NK-92, an immortal line of NK-like cells licensed to NantKwest, Inc.: the response of NK-92 cells that have been transfected with a high-affinity Fc receptor are compared to that of the "wild type" NK-92 which does not express the Fc receptor.[31]
Cytokine-induced NK and Cytotoxic T lymphocyte (CTL) activation
Cytokines play a crucial role in NK cell activation. As these are stress molecules released by cells upon viral infection, they serve to signal to the NK cell the presence of viral pathogens in the affected area. Cytokines involved in NK activation include IL-12, IL-15, IL-18, IL-2, and CCL5. NK cells are activated in response to interferons or macrophage-derived cytokines. They serve to contain viral infections while the adaptive immune response generates antigen-specific cytotoxic T cells that can clear the infection. NK cells work to control viral infections by secreting IFNγ and TNFα. IFNγ activates macrophages for phagocytosis and lysis, and TNFα acts to promote direct NK tumor cell killing. Patients deficient in NK cells prove to be highly susceptible to early phases of herpes virus infection. [Citation needed]
Missing 'self' hypothesis
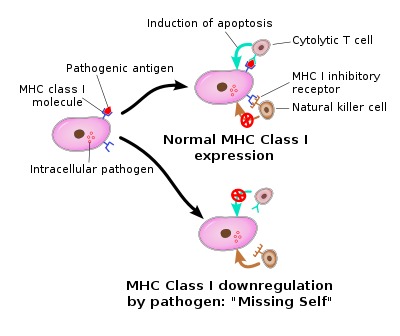
For NK cells to defend the body against viruses and other pathogens, they require mechanisms that enable the determination of whether a cell is infected or not. The exact mechanisms remain the subject of current investigation, but recognition of an "altered self" state is thought to be involved. To control their cytotoxic activity, NK cells possess two types of surface receptors: activating receptors and inhibitory receptors, including killer-cell immunoglobulin-like receptors. Most of these receptors are not unique to NK cells and can be present in some T cell subsets, as well.
The inhibitory receptors recognize MHC class I alleles, which could explain why NK cells preferentially kill cells that possess low levels of MHC class I molecules. This mode of NK cell target interaction is known as "missing-self recognition", a term coined by Klas Kärre and co-workers in the late 90s. MHC class I molecules are the main mechanism by which cells display viral or tumor antigens to cytotoxic T cells. A common evolutionary adaptation to this is seen in both intracellular microbes and tumors: the chronic down-regulation of MHC I molecules, which makes affected cells invisible to T cells, allowing them to evade T cell-mediated immunity. NK cells apparently evolved as an evolutionary response to this adaptation (the loss of the MHC eliminates CD4/CD8 action, so another immune cell evolved to fulfill the function).[32]
Tumor cell surveillance
Natural killer cells often lack antigen-specific cell surface receptors, so are part of innate immunity, i.e. able to react immediately with no prior exposure to the pathogen. In both mice and humans, NKs can be seen to play a role in tumor immunosurveillance by directly inducing the death of tumor cells (NKs act as cytolytic effector lymphocytes), even in the absence of surface adhesion molecules and antigenic peptides. This role of NK cells is critical to immune success particularly because T cells are unable to recognize pathogens in the absence of surface antigens.[2] Tumor cell detection results in activation of NK cells and consequent cytokine production and release.
If tumor cells do not cause inflammation, they will also be regarded as self and will not induce a T cell response. A number of cytokines are produced by NKs, including tumor necrosis factor α (TNFα), IFNγ, and interleukin (IL-10). TNFα and IL-10 act as proinflammatory and immunosuppressors, respectively. The activation of NK cells and subsequent production of cytolytic effector cells impacts macrophages, dendritic cells, and neutrophils, which subsequently enables antigen-specific T and B cell responses. Instead of acting via antigen-specific receptors, lysis of tumor cells by NK cells is mediated by alternative receptors, including NKG2D, NKp44, NKp46, NKp30, and DNAM.[26] NKG2D is a disulfide-linked homodimer which recognizes a number of ligands, including ULBP and MICA, which are typically expressed on tumor cells. The role of dendritic cell—NK cell interface in immunobiology have been studied and defined as critical for the comprehension of the complex immune system.[citation needed]
NK cells, along with macrophages and several other cell types, express the Fc receptor (FcR) molecule (FC-gamma-RIII = CD16), an activating biochemical receptor that binds the Fc portion of IgG class antibodies. This allows NK cells to target cells against which there has been a humoral response and to lyse cells through antibody-dependant cytotoxicity (ADCC). This response depends on the affinity of the Fc receptor expressed on NK cells, which can have high, intermediate, and low affinity for the Fc portion of the antibody. This affinity is determined by the amino acid in position 158 of the protein, which can be phenylalanine (F allele) or valine (V allele). Individuals with high-affinity FcRgammRIII (158 V/V allele) respond better to antibody therapy. This has been shown for lymphoma patients who received the antibody Rituxan. Patients who express the 158 V/V allele had a better antitumor response. Only 15–25% of the population expresses the 158 V/V allele. To determine the ADCC contribution of monoclonal antibodies, NK-92 cells (a "pure" NK cell line) has been transfected with the gene for the high-affinity FcR.
Clearance of senescent cells
Natural killer cells (NK cells) and macrophages play a major role in clearance of senescent cells.[33] Natural killer cells directly kill senescent cells, and produce cytokines which activate macrophages which remove senescent cells.[33]
Natural killer cells can use NKG2D receptors to detect senescent cells, and kill those cells using perforin pore-forming cytolytic protein.[34] CD8+ cytotoxic T-lymphocytes also use NKG2D receptors to detect senescent cells, and promote killing similar to NK cells.[34] For example, in patients with Parkinson's disease, levels of Natural killer cells are elevated as they degrade alpha-synuclein aggregates, destroy senescent neurons, and attenuate the neuroinflammation by leukocytes in the central nervous system.[35]
Adaptive features of NK cells—"memory-like", "adaptive" and memory NK cells
The ability to generate memory cells following a primary infection and the consequent rapid immune activation and response to succeeding infections by the same antigen is fundamental to the role that T and B cells play in the adaptive immune response. For many years, NK cells have been considered to be a part of the innate immune system. However, recently increasing evidence suggests that NK cells can display several features that are usually attributed to adaptive immune cells (e.g. T cell responses) such as dynamic expansion and contraction of subsets, increased longevity and a form of immunological memory, characterized by a more potent response upon secondary challenge with the same antigen.[36][37] In mice, the majority of research was carried out with murine cytomegalovirus (MCMV) and in models of hapten-hypersensitivity reactions. Especially, in the MCMV model, protective memory functions of MCMV-induced NK cells were discovered[38] and direct recognition of the MCMV-ligand m157 by the receptor Ly49 was demonstrated to be crucial for the generation of adaptive NK cell responses.[38] In humans, most studies have focused on the expansion of an NK cell subset carrying the activating receptor NKG2C (KLRC2). Such expansions were observed primarily in response to human cytomegalovirus (HCMV),[39] but also in other infections including Hantavirus, Chikungunya virus, HIV, or viral hepatitis. However, whether these virus infections trigger the expansion of adaptive NKG2C+ NK cells or whether other infections result in re-activation of latent HCMV (as suggested for hepatitis [40]), remains a field of study. Notably, recent research suggests that adaptive NK cells can use the activating receptor NKG2C (KLRC2) to directly bind to human cytomegalovirus-derived peptide antigens and respond to peptide recognition with activation, expansion, and differentiation,[41] a mechanism of responding to virus infections that was previously only known for T cells of the adaptive immune system.
NK cell function in pregnancy
As the majority of pregnancies involve two parents who are not tissue-matched, successful pregnancy requires the mother's immune system to be suppressed. NK cells are thought to be an important cell type in this process.[42] These cells are known as "uterine NK cells" (uNK cells) and they differ from peripheral NK cells. They are in the CD56bright NK cell subset, potent at cytokine secretion, but with low cytotoxic ability and relatively similar to peripheral CD56bright NK cells, with a slightly different receptor profile.[42] These uNK cells are the most abundant leukocytes present in utero in early pregnancy, representing about 70% of leukocytes here, but from where they originate remains controversial.[43]
These NK cells have the ability to elicit cell cytotoxicity in vitro, but at a lower level than peripheral NK cells, despite containing perforin.[44] Lack of cytotoxicity in vivo may be due to the presence of ligands for their inhibitory receptors. Trophoblast cells downregulate HLA-A and HLA-B to defend against cytotoxic T cell-mediated death. This would normally trigger NK cells by missing self recognition; however, these cells survive. The selective retention of HLA-E (which is a ligand for NK cell inhibitory receptor NKG2A) and HLA-G (which is a ligand for NK cell inhibitory receptor KIR2DL4) by the trophoblast is thought to defend it against NK cell-mediated death.[42]
Uterine NK cells have shown no significant difference in women with recurrent miscarriage compared with controls. However, higher peripheral NK cell percentages occur in women with recurrent miscarriages than in control groups.[45]
NK cells secrete a high level of cytokines which help mediate their function. NK cells interact with HLA-C to produce cytokines necessary for trophoblastic proliferation. Some important cytokines they secrete include TNF-α, IL-10, IFN-γ, GM-CSF and TGF-β, among others.[42] For example, IFN-γ dilates and thins the walls of maternal spiral arteries to enhance blood flow to the implantation site.[46]
NK cell evasion by tumor cells
By shedding decoy NKG2D soluble ligands, tumor cells may avoid immune responses. These soluble NKG2D ligands bind to NK cell NKG2D receptors, activating a false NK response and consequently creating competition for the receptor site.[2] This method of evasion occurs in prostate cancer. In addition, prostate cancer tumors can evade CD8 cell recognition due to their ability to downregulate expression of MHC class 1 molecules. This example of immune evasion actually highlights NK cells' importance in tumor surveillance and response, as CD8 cells can consequently only act on tumor cells in response to NK-initiated cytokine production (adaptive immune response).[47]
Excessive NK cells
Experimental treatments with NK cells have resulted in excessive cytokine production, and even septic shock. Depletion of the inflammatory cytokine interferon gamma reversed the effect.[citation needed]
Applications
Anticancer therapy
Tumor-infiltrating NK cells have been reported to play a critical role in promoting drug-induced cell death in human triple-negative breast cancer.[48] Since NK cells recognize target cells when they express nonself HLA antigens (but not self), autologous (patients' own) NK cell infusions have not shown any antitumor effects. Instead, investigators are working on using allogeneic cells from peripheral blood, which requires that all T cells be removed before infusion into the patients to remove the risk of graft versus host disease, which can be fatal. This can be achieved using an immunomagnetic column (CliniMACS). In addition, because of the limited number of NK cells in blood (only 10% of lymphocytes are NK cells), their number needs to be expanded in culture. This can take a few weeks and the yield is donor-dependent.
CAR-NK cells
Chimeric antigen receptors (CARs) are genetically modified receptors targeting cell surface antigens that provide a valuable approach to enhance effector cell efficacy. CARs induce high-affinity binding of effector cells carrying this receptor to cells expressing the target antigen, thereby lowering the threshold for cellular activation and inducing effector functions.[49]
CAR T cells are now a fairly well-known cell therapy. However, wider use is limited by several fundamental problems: The high cost of CAR T cell therapy, which is due to the need to generate specific CAR T cells for each patient; the necessity to use only autologous T cells, due to the high risk of GvHD if allogeneic T cells are used; the inability to reinfuse CAR T cells if the patient relapses or low CAR T cell survival is observed; CAR T therapy also has a high toxicity, mainly due to IFN-γ production and subsequent induction of CRS (cytokine release syndrome) and/or neurotoxicity.[50]
The use of CAR NK cells is not eliminated by the need to generate patient-specific cells, and at the same time, GvHD is not caused by NK cells, thus obviating the need for autologous cells.[51] Toxic effects of CAR T therapy, such as CSR, have not been observed with the use of CAR NK cells. Thus, NK cells are considered an interesting "off-the-shelf" product option. Compared to CAR T cells, CAR NK cells retain unchanged expression of NK cell activating receptors. Thus, NK cells recognize and kill tumor cells even if, due to a tumor-escape strategy on tumor cells, ligand expression for the CAR receptor is downregulated.[50]
NK cells derived from umbilical cord blood have been used to generate CAR.CD19 NK cells. These cells are capable of self-producing the cytokine IL-15, thereby enhancing autocrine/paracrine expression and persistence in vivo. Administration of these modified NK cells is not associated with the development of CSR, neurotoxicity, or GvHD.[49]
The FT596 product is the first “Off-the-Shelf”, universal, and allogenic CAR NK cellular product derived from iPSCs to be authorized for use in clinical studies in the USA.[52] It consists of an anti-CD19 CAR optimized for NK cells with a transmembrane domain for the NKG2D activation receptor, a 2B4 costimulatory domain and a CD3ζ signaling domain. Two additional key components were added. A high-affinity, non-cleavable Fc receptor CD16 (hnCD16) that enables tumor targeting and enhanced antibody-dependent cell cytotoxicity without negative regulation, combined with a therapeutic monoclonal antibody targeting tumor cells and an IL-15/IL-15 receptor fusion protein (IL-15RF) promoting cytokine-independent persistence.[53]
NK-92 cells
A more efficient way to obtain high numbers of NK cells is to expand NK-92 cells, an NK cell line with all the characteristics of highly active blood Natural Killer (NK) cells but with much broader and higher cytotoxicity. NK-92 cells grow continuously in culture and can be expanded to clinical-grade numbers in bags or bioreactors.[54] Clinical studies have shown NK-92 cells to be safe and to exhibit anti-tumor activity in patients with lung or pancreatic cancer, melanoma, and lymphoma.[55][56] Because NK-92 cells originated from a patient with lymphoma, they must be irradiated prior to infusion.[57][58] although efforts are being made to engineer the cells to eliminate the need for irradiation. The irradiated cells maintain full cytotoxicity. NK-92 are allogeneic (from a donor different from the recipient), but in clinical studies have not been shown to elicit significant host reaction.[59][60]
Unmodified NK-92 cells lack CD-16, making them unable to perform antibody-dependent cellular cytotoxicity (ADCC); however, the cells have been engineered to express a high affinity Fc-receptor (CD16A, 158V) genetically linked to IL-2 that is bound to the endoplasmic reticulum (ER).[61][62] These high affinity NK-92 cells can perform ADCC and have greatly expanded therapeutic utility.[63][64][65][66]
NK-92 cells have also been engineered to expressed chimeric antigen receptors (CARs), in an approach similar to that used for T cells. An example of this is an NK-92 derived cell engineered with both a CD16 and an anti-PD-L1 CAR; currently in clinical development for oncology indications.[67][68][69] A clinical grade NK-92 variant that expresses a CAR for HER2 (ErbB2) has been generated[70] and is in a clinical study in patients with HER2 positive glioblastoma.[71] Several other clinical grade clones have been generated expressing the CARs for PD-L1, CD19, HER-2, and EGFR.[72][64] PD-L1 targeted high affinity NK cells have been given to a number of patients with solid tumors in a phase I/II study, which is underway.[73]
NKG2D-Fc fusion protein
In a study at Boston Children's Hospital, in coordination with Dana–Farber Cancer Institute, in which immunocompromised mice had contracted lymphomas from EBV infection, an NK-activating receptor called NKG2D was fused with a stimulatory Fc portion of the EBV antibody. The NKG2D-Fc fusion proved capable of reducing tumor growth and prolonging survival of the recipients. In a transplantation model of LMP1-fueled lymphomas, the NKG2D-Fc fusion proved capable of reducing tumor growth and prolonging survival of the recipients.
In Hodgkin lymphoma, in which the malignant Hodgkin Reed-Sternberg cells are typically HLA class I deficient, immune evasion is in part mediated by skewing towards an exhausted PD-1hi NK cell phenotype, and re-activation of these NK cells appears to be one mechanism of action induced by checkpoint-blockade.[74]
TLR ligands
Signaling through TLR can effectively activate NK cell effector functions in vitro and in vivo. TLR ligands are than potentially able to enhance NK cell effector functions during NK cell anti-tumor immunotherapy.[28]
Trastuzumab is a monoclonal anti-HER2 antibody that is used as a treatment of the HER2+ breast cancer.[75] NK cells are important part of the therapeutical effect of trastzumab as NK cells recognize the antibody coated cancer cells which induces ADCC (antibody-dependent cellular cytotoxicity) reaction. TLR ligand is used as a in addition to trastuzumab as a means to enhance its effect. The polysaccharide krestin, which is extracted from Trametes versicolor, is a potent ligand of TLR-2 and so activates NK cells, induces the production of IFNg and enhances the ADCC caused by recognition of trastuzumab-coated cells.[76]
Stimulation of TLR-7 induces the expression of IFN type I and other pro-inflammatory cytokines like IL-1b, IL-6 and IL-12. Mice suffering with NK cell-sensitive lymphoma RMA-S were treated with SC1 molecule. SC1 is novel small-molecule TLR-7 agonist and its repeated administration reportedly activated NK cells in TLR-7- and IFN type I- dependent manner thus reversing the NK cell anergy which ultimately lead to lysis of the tumor.[77]
VTX-2337 is a selective TLR-8 agonist and together with monoclonal antibody cetuximab it was used as a potential therapy for the treatment of recurrent or metastatic SCCHN. The results show that NK cells has become more reactive to the treatment with cetuximab antibody upon pretreatment with VTX-2337. This indicates that the stimulation of TLR-8 and subsequent activation of inflammasome enhances the CD-16 mediated ADCC reaction in patients treated with cetuximab antibody.[78]
NK cells play a role in controlling HIV-1 infection. TLR are potent enhancers of innate antiviral immunity and potentially can reverse HIV-1 latency. Incubation of peripheral blood mononuclear cells with novel potent TLR-9 ligand MGN1703 have resulted in enhancement of NK cell effector functions, thus significantly inhibiting the spread of HIV-1 in culture of autologous CD4+ T-cells. The stimulation of TLR-9 in NK cells induced strong antiviral innate immune response, the increase in HIV-1 transcription (indicating the reverse in latency of the virus) and it also boosted the NK cell-mediated suppression of HIV-1 infections in autologous CD4+ T cells.[79]
New findings
Innate resistance to HIV
Recent research suggests specific KIR-MHC class I gene interactions might control innate genetic resistance to certain viral infections, including HIV and its consequent development of AIDS.[6] Certain HLA allotypes have been found to determine the progression of HIV to AIDS; an example is the HLA-B57 and HLA-B27 alleles, which have been found to delay progression from HIV to AIDS. This is evident because patients expressing these HLA alleles are observed to have lower viral loads and a more gradual decline in CD4+ T cells numbers. Despite considerable research and data collected measuring the genetic correlation of HLA alleles and KIR allotypes, a firm conclusion has not yet been drawn as to what combination provides decreased HIV and AIDS susceptibility.
NK cells can impose immune pressure on HIV, which had previously been described only for T cells and antibodies.[80] HIV mutates to avoid NK cell detection.[80]
Tissue-resident NK cells
Most of our current knowledge is derived from investigations of mouse splenic and human peripheral blood NK cells. However, in recent years tissue-resident NK cell populations have been described.[81][82] These tissue-resident NK cells share transcriptional similarity to tissue-resident memory T cells described previously. However, tissue-resident NK cells are not necessarily of the memory phenotype, and in fact, majority of the tissue-resident NK cells functionally immature.[83] These specialized NK-cell subsets can play a role in organ homeostasis. For example, NK cells are enriched in the human liver with a specific phenotype and take part in the control of liver fibrosis.[84][85] Tissue-resident NK cells have also been identified in sites like bone marrow, spleen and more recently, in lung, intestines and lymph nodes. In these sites, tissue-resident NK cells may act as reservoir for maintaining immature NK cells in humans throughout life.[83]
Adaptive NK cells against leukemia targets
Natural killer cells are being investigated as an emerging treatment for patients with acute myeloid leukemia (AML), and cytokine-induced memory-like NK cells have shown promise with their enhanced antileukemia functionality.[86] It has been shown that this kind of NK cell has enhanced interferon-γ production and cytotoxicity against leukemia cell lines and primary AML blasts in patients.[86] During a phase 1 clinical trial, five out of nine patients exhibited clinical responses to the treatment, and four patients experienced a complete remission, which suggests that these NK cells have major potential as a successful translational immunotherapy approach for patients with AML in the future.[86]
See also
- Active hexose correlated compound
- Granzymes
- Hematopoiesis
- Immune system
- Interleukin
- Lymphatic system
- List of distinct cell types in the adult human body
References
- ↑ "Human NK cells: From development to effector functions". Innate Immunity 27 (3): 212–229. April 2021. doi:10.1177/17534259211001512. PMID 33761782.
- ↑ 2.0 2.1 2.2 "Innate or adaptive immunity? The example of natural killer cells". Science 331 (6013): 44–49. January 2011. doi:10.1126/science.1198687. PMID 21212348. Bibcode: 2011Sci...331...44V.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Deciphering Natural Killer Cell Homeostasis". Frontiers in Immunology 11: 812. 2020. doi:10.3389/fimmu.2020.00812. PMID 32477340.
- ↑ 4.0 4.1 "Innate lymphocytes-lineage, localization and timing of differentiation". Cellular & Molecular Immunology 16 (7): 627–633. July 2019. doi:10.1038/s41423-019-0211-7. PMID 30804475.
- ↑ "Development and differentiation of early innate lymphoid progenitors". The Journal of Experimental Medicine 215 (1): 249–262. January 2018. doi:10.1084/jem.20170832. PMID 29183988.
- ↑ 6.0 6.1 6.2 6.3 "Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS". Journal of Leukocyte Biology 84 (1): 1–26. July 2008. doi:10.1189/jlb.0907650. PMID 18388298.
- ↑ "Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46". Proceedings of the National Academy of Sciences of the United States of America 104 (9): 3384–3389. February 2007. doi:10.1073/pnas.0609692104. PMID 17360655. Bibcode: 2007PNAS..104.3384W.
- ↑ "p46, a novel natural killer cell-specific surface molecule that mediates cell activation". The Journal of Experimental Medicine 186 (7): 1129–1136. October 1997. doi:10.1084/jem.186.7.1129. PMID 9314561.
- ↑ "Cellular liaisons of natural killer lymphocytes in immunology and immunotherapy of cancer". Expert Opinion on Biological Therapy 7 (5): 599–615. May 2007. doi:10.1517/14712598.7.5.599. PMID 17477799.
- ↑ How to trigger a killer: modulation of natural killer cell reactivity on many levels. Advances in Immunology. 124. 2014. pp. 137–70. doi:10.1016/B978-0-12-800147-9.00005-4. ISBN 9780128001479.
- ↑ Perera Molligoda Arachchige, Arosh S (2022-03-25). "NK cell-based therapies for HIV infection: Investigating current advances and future possibilities" (in en). Journal of Leukocyte Biology 111 (4): 921–931. doi:10.1002/JLB.5RU0821-412RR. ISSN 0741-5400. https://academic.oup.com/jleukbio/article/111/4/921/6885120.
- ↑ Arachchige, Arosh S. Perera Molligoda (2021). "A universal CAR-NK cell approach for HIV eradication" (in en). AIMS Allergy and Immunology 5 (3): 192–194. doi:10.3934/Allergy.2021015. ISSN 2575-615X. http://www.aimspress.com/rticle/doi/10.3934/Allergy.2021015.
- ↑ "Antigenicity of carcinogen-induced and spontaneous tumours in inbred mice". British Journal of Cancer 20 (4): 831–837. December 1966. doi:10.1038/bjc.1966.95. PMID 5964614.
- ↑ "Natural killer cells: artifact to reality: an odyssey in biology". Cancer and Metastasis Reviews 2 (4): 323–336. 1983. doi:10.1007/BF00048565. PMID 6375859.
- ↑ ""Natural" killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell". European Journal of Immunology 5 (2): 117–121. February 1975. doi:10.1002/eji.1830050209. PMID 1086218.
- ↑ "Cytotoxic lymphocytes from normal donors. A functional marker of human non-T lymphocytes". Clinical and Experimental Immunology 21 (2): 226–235. August 1975. PMID 810282.
- ↑ "Surface markers on human b and t lymphocytes. VI. Cytotoxicity against cell lines as a functional marker for lymphocyte subpopulations". International Journal of Cancer 15 (4): 596–605. April 1975. doi:10.1002/ijc.2910150409. PMID 806545.
- ↑ "Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells". International Journal of Cancer 16 (2): 230–239. August 1975. doi:10.1002/ijc.2910160205. PMID 1080480.
- ↑ "Natural cytotoxic reactivity of human lymphocytes against a myeloid cell line: characterization of effector cells". Journal of Immunology 118 (1): 355–361. January 1977. doi:10.4049/jimmunol.118.1.355. PMID 299761.
- ↑ "Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity". Journal of Clinical Immunology 1 (1): 51–63. January 1981. doi:10.1007/BF00915477. PMID 7334070.
- ↑ "Isolation of human NK cells by density gradient centrifugation". Journal of Immunological Methods 36 (3–4): 285–291. 1980. doi:10.1016/0022-1759(80)90133-7. PMID 7430655.
- ↑ "Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance". Cancers 12 (6): 1553. June 2020. doi:10.3390/cancers12061553. PMID 32545516.
- ↑ 23.0 23.1 23.2 23.3 "Natural killer cells in cancer biology and therapy". Molecular Cancer 19 (1): 120. August 2020. doi:10.1186/s12943-020-01238-x. PMID 32762681.
- ↑ Lee, Mercede; Bell, Charles JM; Rubio García, Arcadio (2023). "CD56bright natural killer cells preferentially kill proliferating CD4+ T cells". Discovery Immunology: kyad012. doi:10.1093/discim/kyad012. https://academic.oup.com/discovimmunology/advance-article/doi/10.1093/discim/kyad012/7241354.
- ↑ 25.0 25.1 "Flattening the COVID-19 Curve With Natural Killer Cell Based Immunotherapies". Frontiers in Immunology 11: 1512. 2020. doi:10.3389/fimmu.2020.01512. PMID 32655581.
- ↑ 26.0 26.1 "Potential role of NK cells in the induction of immune responses: implications for NK cell-based immunotherapy for cancers and viral infections". International Reviews of Immunology 27 (3): 93–110. 2008. doi:10.1080/08830180801911743. PMID 18437601.
- ↑ "Toll-Like Receptors and Natural Killer Cells" (in en). Toll-like Receptors. IntechOpen. 2020-01-15. doi:10.5772/intechopen.86393. ISBN 978-1-78984-523-5. https://www.intechopen.com/books/toll-like-receptors/toll-like-receptors-and-natural-killer-cells. Retrieved 2023-06-15.
- ↑ 28.0 28.1 "Toll-Like Receptors in Natural Killer Cells and Their Application for Immunotherapy". Journal of Immunology Research 2020: 2045860. 2020. doi:10.1155/2020/2045860. PMID 32377528.
- ↑ "TLR/NCR/KIR: Which One to Use and When?". Frontiers in Immunology 5: 105. 2014. doi:10.3389/fimmu.2014.00105. PMID 24678311.
- ↑ "Toll-like receptors in ischaemia and its potential role in the pathophysiology of muscle damage in critical limb ischaemia". Cardiology Research and Practice 2012: 121237. 2012. doi:10.1155/2012/121237. PMID 22454775.
- ↑ "New aspects of natural-killer-cell surveillance and therapy of cancer". Nature Reviews. Cancer 2 (11): 850–861. November 2002. doi:10.1038/nrc928. PMID 12415255.
- ↑ "Viral modulation of NK cell immunity". Nature Reviews. Microbiology 3 (1): 59–69. January 2005. doi:10.1038/nrmicro1066. PMID 15608700.
- ↑ 33.0 33.1 "Senescent cells: Living or dying is a matter of NK cells". Journal of Leukocyte Biology 105 (6): 1275–1283. June 2019. doi:10.1002/JLB.MR0718-299R. PMID 30811627.
- ↑ 34.0 34.1 "Senescent cell clearance by the immune system: Emerging therapeutic opportunities". Seminars in Immunology 40: 101275. December 2018. doi:10.1016/j.smim.2019.04.003. PMID 31088710.
- ↑ "The role of natural killer cells in Parkinson's disease". Experimental & Molecular Medicine 52 (9): 1517–1525. September 2020. doi:10.1038/s12276-020-00505-7. PMID 32973221.
- ↑ "Memory of infections: an emerging role for natural killer cells". PLOS Pathogens 9 (9): e1003548. September 2013. doi:10.1371/journal.ppat.1003548. PMID 24086127.
- ↑ "Natural killer cells: NK cells stroll down the memory lane". Immunology and Cell Biology 87 (4): 261–263. 2009. doi:10.1038/icb.2009.10. PMID 19290015.
- ↑ 38.0 38.1 "Adaptive immune features of natural killer cells". Nature 457 (7229): 557–561. January 2009. doi:10.1038/nature07665. PMID 19136945. Bibcode: 2009Natur.457..557S.
- ↑ "Imprint of human cytomegalovirus infection on the NK cell receptor repertoire". Blood 104 (12): 3664–3671. December 2004. doi:10.1182/blood-2004-05-2058. PMID 15304389.
- ↑ "Cytomegalovirus-Driven Adaptive-Like Natural Killer Cell Expansions Are Unaffected by Concurrent Chronic Hepatitis Virus Infections". Frontiers in Immunology 8 (8): 525. 2017. doi:10.3389/fimmu.2017.00525. PMID 28533779.
- ↑ "Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells". Nature Immunology 19 (5): 453–463. May 2018. doi:10.1038/s41590-018-0082-6. PMID 29632329.
- ↑ 42.0 42.1 42.2 42.3 "Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua". Placenta 31 (Suppl): S87–S92. March 2010. doi:10.1016/j.placenta.2009.12.022. PMID 20061017.
- ↑ "Immune cells in the placental bed". The International Journal of Developmental Biology 54 (2–3): 281–294. 2010. doi:10.1387/ijdb.082763jb. PMID 19876837.
- ↑ "Human decidual NK cells form immature activating synapses and are not cytotoxic". Proceedings of the National Academy of Sciences of the United States of America 102 (43): 15563–15568. October 2005. doi:10.1073/pnas.0507835102. PMID 16230631. Bibcode: 2005PNAS..10215563K.
- ↑ "Natural killer cells in female infertility and recurrent miscarriage: a systematic review and meta-analysis". Human Reproduction Update 20 (3): 429–438. 2013. doi:10.1093/humupd/dmt056. PMID 24285824.
- ↑ "Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy". The Journal of Experimental Medicine 192 (2): 259–270. July 2000. doi:10.1084/jem.192.2.259. PMID 10899912.
- ↑ "T cell- and B cell-independent adaptive immunity mediated by natural killer cells". Nature Immunology 7 (5): 507–516. May 2006. doi:10.1038/ni1332. PMID 16617337.
- ↑ "Nanoengineered Disruption of Heat Shock Protein 90 Targets Drug-Induced Resistance and Relieves Natural Killer Cell Suppression in Breast Cancer". Cancer Research 80 (23): 5355–5366. December 2020. doi:10.1158/0008-5472.CAN-19-4036. PMID 33077554.
- ↑ 49.0 49.1 "NK cells and ILCs in tumor immunotherapy". Molecular Aspects of Medicine 80: 100870. August 2021. doi:10.1016/j.mam.2020.100870. PMID 32800530.
- ↑ 50.0 50.1 "NK Cell-Based Immunotherapy for Hematological Malignancies". Journal of Clinical Medicine 8 (10): 1702. October 2019. doi:10.3390/jcm8101702. PMID 31623224.
- ↑ "Next generation natural killer cells for cancer immunotherapy: the promise of genetic engineering". Current Opinion in Immunology 51: 146–153. April 2018. doi:10.1016/j.coi.2018.03.013. PMID 29605760.
- ↑ "FT596: Translation of First-of-Kind Multi-Antigen Targeted Off-the-Shelf CAR-NK Cell with Engineered Persistence for the Treatment of B Cell Malignancies". Blood 134 (Supplement_1): 301. 2019-11-13. doi:10.1182/blood-2019-129319. ISSN 0006-4971.
- ↑ "Initial Clinical Activity of FT596, a First-in-Class, Multi-Antigen Targeted, Off-the-Shelf, iPSC-Derived CD19 CAR NK Cell Therapy in Relapsed/Refractory B-Cell Lymphoma" (in en). Blood 136 (Supplement 1): 8. 2020-11-05. doi:10.1182/blood-2020-141606. ISSN 0006-4971. https://ashpublications.org/blood/article/136/Supplement%201/8/472050/Initial-Clinical-Activity-of-FT596-a-First-in.
- ↑ Gong JH, Maki G, Klingemann HG (April 1994). "Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells". Leukemia. 8 (4): 652–8. PMID 8152260.
- ↑ "Infusion of the allogeneic cell line NK-92 in patients with advanced renal cell cancer or melanoma: a phase I trial". Cytotherapy 10 (6): 625–632. 2008. doi:10.1080/14653240802301872. PMID 18836917.
- ↑ "Cellular immunotherapy of malignancies using the clonal natural killer cell line NK-92". Journal of Hematotherapy & Stem Cell Research 10 (4): 535–544. August 2001. doi:10.1089/15258160152509145. PMID 11522236.
- ↑ "Factors regulating the cytotoxic activity of the human natural killer cell line, NK-92". Journal of Hematotherapy & Stem Cell Research 10 (3): 369–383. June 2001. doi:10.1089/152581601750288975. PMID 11454312.
- ↑ "Optimizing NK-92 serial killers: gamma irradiation, CD95/Fas-ligation, and NK or LAK attack limit cytotoxic efficacy". Journal of Translational Medicine 20 (1): 151. April 2022. doi:10.1186/s12967-022-03350-6. PMID 35366943.
- ↑ "A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy". Oncotarget 8 (51): 89256–89268. October 2017. doi:10.18632/oncotarget.19204. PMID 29179517.
- ↑ "Treatment of patients with advanced cancer with the natural killer cell line NK-92". Cytotherapy 15 (12): 1563–1570. December 2013. doi:10.1016/j.jcyt.2013.06.017. PMID 24094496.
- ↑ "An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele". Oncotarget 7 (52): 86359–86373. December 2016. doi:10.18632/oncotarget.13411. PMID 27861156.
- ↑ "Expression of a Recombinant High Affinity IgG Fc Receptor by Engineered NK Cells as a Docking Platform for Therapeutic mAbs to Target Cancer Cells". Frontiers in Immunology 9: 2873. 2018. doi:10.3389/fimmu.2018.02873. PMID 30574146.
- ↑ Klingemann H. “Engineered, Off the Shelf, NK Cell Lines for Targeted Cancer Immuno-therapy.” Frontiers in Cancer Immunotherapy, NYAS April 26–27, 2018. www.nyas.org/Immunotherapy2018
- ↑ 64.0 64.1 "Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity". Oncoimmunology 2 (10): e26527. October 2013. doi:10.4161/onci.26527. PMID 24404423.
- ↑ "NK Cell–Targeting Strategies Come into Their Own". 16 April 2021. https://www.onclive.com/view/nk-cell-targeting-strategies-come-into-their-own.
- ↑ "The emerging role of off-the-shelf engineered natural killer cells in targeted cancer immunotherapy". Molecular Therapy Oncolytics 23: 266–276. December 2021. doi:10.1016/j.omto.2021.10.001. PMID 34761106.
- ↑ "Open-Label Phase 1 Study of PD-L1 t-haNK in Subjects with Locally Advanced or Metastatic Solid Cancers". 9 May 2022. https://www.clinicaltrials.gov/ct2/show/NCT04050709?term=t-haNK&cond=cancer&draw=2&rank=1.
- ↑ "Tumor control via targeting PD-L1 with chimeric antigen receptor modified NK cells". eLife 9. July 2020. doi:10.7554/eLife.54854. PMID 32633234.
- ↑ "PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations". Journal for Immunotherapy of Cancer 8 (1): e000450. May 2020. doi:10.1136/jitc-2019-000450. PMID 32439799.
- ↑ "ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma". Journal of the National Cancer Institute 108 (5). May 2016. doi:10.1093/jnci/djv375. PMID 26640245.
- ↑ "CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy". Frontiers in Immunology 10: 2683. 2019. doi:10.3389/fimmu.2019.02683. PMID 31798595.
- ↑ "CD19-CAR engineered NK-92 cells are sufficient to overcome NK cell resistance in B-cell malignancies". Journal of Cellular and Molecular Medicine 20 (7): 1287–1294. July 2016. doi:10.1111/jcmm.12810. PMID 27008316.
- ↑ "ImmunityBio Announces NIH-Led Research Affirming that PD-L1 T-haNK Therapy Overcomes T-Cell Escape in Multiple Types of Resistant Tumors". 22 March 2021. https://immunitybio.com/immunitybio-announces-nih-led-research-affirming-that-pd-l1-t-hank-therapy-overcomes-t-cell-escape-in-multiple-types-of-resistant-tumors/.
- ↑ "Immune evasion via PD-1/PD-L1 on NK cells and monocyte/macrophages is more prominent in Hodgkin lymphoma than DLBCL". Blood 131 (16): 1809–1819. April 2018. doi:10.1182/blood-2017-07-796342. PMID 29449276.
- ↑ "Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer". The Oncologist 11 (Suppl 1): 4–12. 2006-09-01. doi:10.1634/theoncologist.11-90001-4. PMID 16971734.
- ↑ "TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy". Clinical Cancer Research 17 (21): 6742–6753. November 2011. doi:10.1158/1078-0432.CCR-11-1142. PMID 21918170.
- ↑ "A novel TLR7 agonist reverses NK cell anergy and cures RMA-S lymphoma-bearing mice". Oncoimmunology 5 (7): e1189051. July 2016. doi:10.1080/2162402X.2016.1189051. PMID 27622045.
- ↑ "Coordinated Activation of Toll-Like Receptor8 (TLR8) and NLRP3 by the TLR8 Agonist, VTX-2337, Ignites Tumoricidal Natural Killer Cell Activity". PLOS ONE 11 (2): e0148764. 2016. doi:10.1371/journal.pone.0148764. PMID 26928328. Bibcode: 2016PLoSO..1148764D.
- ↑ "A Novel Toll-Like Receptor 9 Agonist, MGN1703, Enhances HIV-1 Transcription and NK Cell-Mediated Inhibition of HIV-1-Infected Autologous CD4+ T Cells". Journal of Virology 90 (9): 4441–4453. May 2016. doi:10.1128/JVI.00222-16. PMID 26889036.
- ↑ 80.0 80.1 "HIV-1 adaptation to NK-cell-mediated immune pressure". Nature 476 (7358): 96–100. August 2011. doi:10.1038/nature10237. PMID 21814282.
- ↑ "Tissue-resident natural killer cells". Cold Spring Harbor Symposia on Quantitative Biology 78: 149–156. 2013-01-01. doi:10.1101/sqb.2013.78.020354. PMID 24584057.
- ↑ "Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells". eLife 3: e01659. January 2014. doi:10.7554/elife.01659. PMID 24714492.
- ↑ 83.0 83.1 "Tissue Determinants of Human NK Cell Development, Function, and Residence". Cell 180 (4): 749–763.e13. February 2020. doi:10.1016/j.cell.2020.01.022. PMID 32059780.
- ↑ "Human liver-resident CD56(bright)/CD16(neg) NK cells are retained within hepatic sinusoids via the engagement of CCR5 and CXCR6 pathways". Journal of Autoimmunity 66: 40–50. January 2016. doi:10.1016/j.jaut.2015.08.011. PMID 26330348.
- ↑ "Natural Killer Cells and Liver Fibrosis" (in English). Frontiers in Immunology 7: 19. 2016. doi:10.3389/fimmu.2016.00019. PMID 26858722.
- ↑ 86.0 86.1 86.2 "Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia". Science Translational Medicine 8 (357): 357ra123. September 2016. doi:10.1126/scitranslmed.aaf2341. PMID 27655849.
Further reading
- "Human NK cells: From development to effector functions". Innate Immunity 27 (3): 212–229. April 2021. doi:10.1177/17534259211001512. PMID 33761782.
- Cellular and Molecular Immunology. Saunders. 2003.
- How the Immune System Works (2nd ed.). Blackwell Publishing. 2003.
- Immunobiology: The Immune System In Health And Disease (5th ed.). Garland Science. 2001. ISBN 0-8153-3642-X. https://www.ncbi.nlm.nih.gov/books/NBK10757/.
- Kuby Immunology (6th ed.). New York: W.H. Freeman and Company.
External links
- Natural+Killer+Cells at the US National Library of Medicine Medical Subject Headings (MeSH)
 |
