Medicine:Tumoral calcinosis
| Tumoral calcinosis | |
|---|---|
 | |
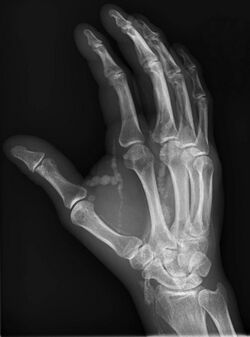
| Hand radiograph showing tumoral calcinosis, PA radiograph of the right hand showing tumoral calcinosis-like metastatic calcification in a patient on dialysis. Dialysis alters calcium phosphate product (>70). Idiopathic tumoral calcinosis is autosomal dominant and is not associated with dialysis. Note the premature arterial calcification which is a clue that this is a renal patient. Vascular calcification contributes to an increase in morbidity. |
Tumoral calcinosis is a rare condition in which there is calcium deposition in the soft tissue in periarticular location, around joints, outside the joint capsule.[1] They are frequently (0.5–3%) seen in patients undergoing renal dialysis. Clinically also known as hyperphosphatemic familial tumoral calcinosis (HFTC), is often caused by genetic mutations in genes that regulate phosphate physiology in the body (leading to too much phosphate (hyperphosphatemia)). Best described genes that harbour mutations in humans are FGF-23,[2] Klotho (KL),[3] or GALNT3.[4] A zebrafish animal model with reduced GALNT3 expression also showed HFTC-like phenotype,[5] indicating an evolutionary conserved mechanism that is involved in developing tumoral calcinosis.
Clinical features
The name indicates calcinosis (calcium deposition) which resembles tumor (like a new growth). They are not true neoplasms – they don't have dividing cells. They are just deposition of inorganic calcium with serum exudate. Children and adolescents (6 to 25 years) are the most commonly affected. The symptom that the accumulations cause is not pain but swelling around joints. They have propensity to enlarge progressively and ulcerate the overlying skin and extrude. They are most common around shoulders, hips and elbows. Laboratory evaluation reveal normal serum calcium levels and hyperphosphatemia. Rarely ALP (alkaline phosphatase – an enzyme active at sites of bone formation) may be elevated. Treatment is normalization of serum phosphate levels and resection of lesions. Surgical removal should be complete and if part of it is left, recurrence is likely to occur. Cutting through the excised calcium deposition reveals semifluid calcium suspension in albumin encapsulated by fibrous tissue.
Additional images
References
- ↑ "Orthobullets". http://www.orthobullets.com/pathology/8077/tumoral-calcinosis. Retrieved 27 October 2014.
- ↑ Araya, Kaori; Fukumoto, Seiji; Backenroth, Rebecca; Takeuchi, Yasuhiro; Nakayama, Kounosuke; Ito, Nobuaki; Yoshii, Nozomi; Yamazaki, Yuji et al. (2005-10-01). "A Novel Mutation in Fibroblast Growth Factor 23 Gene as a Cause of Tumoral Calcinosis" (in en). The Journal of Clinical Endocrinology & Metabolism 90 (10): 5523–5527. doi:10.1210/jc.2005-0301. ISSN 0021-972X. PMID 16030159.
- ↑ Ichikawa, Shoji; Imel, Erik A.; Kreiter, Mary L.; Yu, Xijie; Mackenzie, Donald S.; Sorenson, Andrea H.; Goetz, Regina; Mohammadi, Moosa et al. (2007-09-04). "A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis" (in en). Journal of Clinical Investigation 117 (9): 2684–2691. doi:10.1172/JCI31330. ISSN 0021-9738. PMID 17710231.
- ↑ Topaz, Orit; Shurman, Daniel L; Bergman, Reuven; Indelman, Margarita; Ratajczak, Paulina; Mizrachi, Mordechai; Khamaysi, Ziad; Behar, Doron et al. (June 2004). "Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis" (in en). Nature Genetics 36 (6): 579–581. doi:10.1038/ng1358. ISSN 1061-4036. PMID 15133511.
- ↑ Stevenson, Nicola L.; Bergen, Dylan J. M.; Skinner, Roderick E. H.; Kague, Erika; Martin-Silverstone, Elizabeth; Robson Brown, Kate A.; Hammond, Chrissy L.; Stephens, David J. (2017-12-15). "Giantin-knockout models reveal a feedback loop between Golgi function and glycosyltransferase expression" (in en). Journal of Cell Science 130 (24): 4132–4143. doi:10.1242/jcs.212308. ISSN 0021-9533. PMID 29093022.
External links
 |