Nanomesh
The nanomesh is an inorganic nanostructured two-dimensional material, similar to graphene. It was discovered in 2003 at the University of Zurich, Switzerland.[1]
It consists of a single layer of boron (B) and nitrogen (N) atoms, which forms by self-assembly into a highly regular mesh after high-temperature exposure of a clean rhodium[1] or ruthenium[2] surface to borazine under ultra-high vacuum.
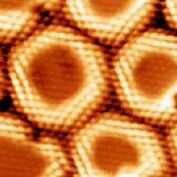
The nanomesh looks like an assembly of hexagonal pores[3] (see right image) at the nanometer (nm) scale. The distance between two pore centers is only 3.2 nm, whereas each pore has a diameter of about 2 nm and is 0.05 nm deep. The lowest regions bind strongly to the underlying metal, while the wires[3] (highest regions) are only bound to the surface through strong cohesive forces within the layer itself.
The boron nitride nanomesh is not only stable under vacuum,[1] air[4] and some liquids,[5][6] but also up to temperatures of 796 °C (1070 K).[1] In addition it shows the extraordinary ability to trap molecules[5] and metallic clusters,[2] which have similar sizes to the nanomesh pores, forming a well-ordered array. These characteristics may provide applications of the material in areas like, surface functionalisation, spintronics, quantum computing and data storage media like hard drives.
Structure

h-BN nanomesh is a single sheet of hexagonal boron nitride, which forms on substrates like rhodium Rh(111) or ruthenium Ru(0001) crystals by a self-assembly process.
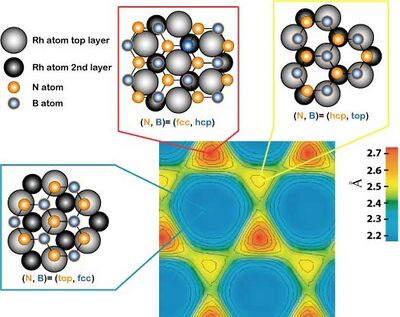
The unit cell of the h-BN nanomesh consists of 13x13 BN or 12x12 Rh atoms with a lattice constant of 3.2 nm. In a cross-section it means that 13 boron or nitrogen atoms are sitting on 12 rhodium atoms. This implies a modification of the relative positions of each BN towards the substrate atoms within a unit cell, where some bonds are more attractive or repulsive than other (site selective bonding), what induces the corrugation of the nanomesh (see right image with pores and wires).
The nanomesh corrugation amplitude of 0.05 nm causes a strong effect on the electronic structure, where two distinct BN regions are observed. They are easily recognized in the lower right image, which is a scanning tunneling microscopy (STM) measurement, as well as in the lower left image representing a theoretical calculation of the same area. A strongly bounded region assigned to the pores is visible in blue in the left image below (center of bright rings in the right image) and a weakly bound region assigned to the wires appears yellow-red in the left image below (area in-between rings in the right image).
See [1][2][4][5][7] for more details.
Properties
The nanomesh is stable under a wide range of environments like air, water and electrolytes among others. It is also temperature resistant since it does not decompose in temperatures up to 1275K under a vacuum. In addition to these exceptional stabilities, the nanomesh shows the extraordinary ability to act as a scaffold for metallic nanoclusters and to trap molecules forming a well-ordered array.
In the case of gold (Au), its evaporation on the nanomesh leads to formation of well-defined round Au nanoparticles, which are centered at the nanomesh pores.
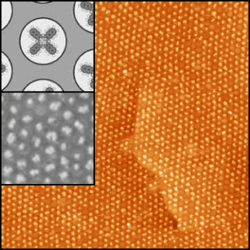
The STM figure on the right shows Naphthalocyanine (Nc) molecules, which were vapor-deposited onto the nanomesh. These planar molecules have a diameter of about 2 nm, whose size is comparable to that of the nanomesh pores (see upper inset). It is spectacularly visible how the molecules form a well-ordered array with the periodicity of the nanomesh (3.22 nm). The lower inset shows a region of this substrate with higher resolution, where individual molecules are trapped inside the pores. In addition, the molecules seem to keep their native conformation, what means that their functionality is kept, which is nowadays a challenge in nanoscience.
Such systems with wide spacing between individual molecules/clusters and negligible intermolecular interactions might be interesting for applications such as molecular electronics and memory elements, in photochemistry or in optical devices.
See [2][5][6] for more detailed information.
Preparation and analysis

Well-ordered nanomeshes are grown by thermal decomposition of borazine (HBNH)3, a colorless substance that is liquid at room temperature. The nanomesh results after exposing the atomically clean Rh(111) or Ru(0001) surface to borazine by chemical vapor deposition (CVD).
The substrate is kept at a temperature of 796 °C (1070 K) when borazine is introduced in the vacuum chamber at a dose of about 40 L (1 Langmuir = 10−6 torr sec). A typical borazine vapor pressure inside the ultrahigh vacuum chamber during the exposure is 3x10−7 mbar.
After cooling down to room temperature, the regular mesh structure is observed using different experimental techniques. Scanning tunneling microscopy (STM) gives a direct look on the local real space structure of the nanomesh, while low energy electron diffraction (LEED) gives information about the surface structures ordered over the whole sample. Ultraviolet photoelectron spectroscopy (UPS) gives information about the electronic states in the outermost atomic layers of a sample, i.e. electronic information of the top substrate layers and the nanomesh.
See also
Other forms
CVD of borazine on other substrates has not led so far to the formation of a corrugated nanomesh. A flat BN layer is observed on nickel[8] and palladium,[9][10] whereas stripped structures appear on molybdenum[11] instead.
References and notes
- ↑ 1.0 1.1 1.2 1.3 1.4 M. Corso; Auwärter, Willi; Muntwiler, Matthias; Tamai, Anna et al. (2004). "Boron Nitride Nanomesh". Science 303 (5655): 217–220. doi:10.1126/science.1091979. PMID 14716010. Bibcode: 2004Sci...303..217C.
- ↑ 2.0 2.1 2.2 2.3 A. Goriachko; He, Y; Knapp, M; Over, H et al. (2007). "Self-assembly of a hexagonal boron nitride nanomesh on Ru(0001)". Langmuir 23 (6): 2928–2931. doi:10.1021/la062990t. PMID 17286422.
- ↑ 3.0 3.1 In the literature different words referring to similar concepts can be found. Below is a summary of them:
- Pores, apertures, holes: areas of the nanomesh laying the closest to the underlying substrate due to a strong attraction. They form depressions, which are 0.05 nm deep and have a hexagonal shape.
- Wires: areas of the nanomesh referring to the border of the pores, which lay the farer away to the underlying substrate and therefore represent the upper part of the nanomesh.
- ↑ 4.0 4.1 O. Bunk; Corso, M; Martoccia, D; Herger, R et al. (2007). "Surface X-ray diffraction study of boron-nitride nanomesh in air". Surf. Sci. 601 (2): L7–L10. doi:10.1016/j.susc.2006.11.018. Bibcode: 2007SurSc.601L...7B. https://www.dora.lib4ri.ch/psi/islandora/object/psi%3A18158.
- ↑ 5.0 5.1 5.2 5.3 S. BernerExpression error: Unrecognized word "etal". (2007). "Boron Nitride Nanomesh: Functionality from a Corrugated Monolayer". Angew. Chem. Int. Ed. 46 (27): 5115–5119. doi:10.1002/anie.200700234. PMID 17538919.
- ↑ 6.0 6.1 R. Widmer; Berner, S; Groning, O; Brugger, T et al. (2007). "Electrolytic in situ STM investigation of h-BN-Nanomesh". Electrochem. Commun. 9 (10): 2484–2488. doi:10.1016/j.elecom.2007.07.019.
- ↑ R. Laskowski; Blaha, Peter; Gallauner, Thomas; Schwarz, Karlheinz (2007). "Single layer model of the h-BN nanomesh on the Rh(111) surface". Phys. Rev. Lett. 98 (10): 106'802. doi:10.1103/PhysRevLett.98.106802. PMID 17358554. Bibcode: 2007PhRvL..98j6802L.
- ↑ T. Greber; Brandenberger, Louis; Corso, Martina; Tamai, Anna et al. (2006). "Single layer hexagonal boron nitride films on Ni(110)". E-J. Surf. Sci. Nanotech. 4: 410. doi:10.1380/ejssnt.2006.410. http://joi.jlc.jst.go.jp/JST.JSTAGE/ejssnt/2006.410.
- ↑ M. Corso; Greber, Thomas; Osterwalder, Jürg (2005). "h-BN on Pd(110): a tunable system for selfassembled nanostructures?". Surf. Sci. 577 (2–3): L78. doi:10.1016/j.susc.2005.01.015. Bibcode: 2005SurSc.577L..78C.
- ↑ M. Morscher; Corso, M.; Greber, T.; Osterwalder, J. (2006). "Formation of single layer h-BN on Pd(111)". Surf. Sci. 600 (16): 3280–3284. doi:10.1016/j.susc.2006.06.016. Bibcode: 2006SurSc.600.3280M.
- ↑ M. Allan; Berner, Simon; Corso, Martina; Greber, Thomas et al. (2007). "Tunable self-assembly of one-dimensional nanostructures with orthogonal directions". Nanoscale Res. Lett. 2 (2): 94–99. doi:10.1007/s11671-006-9036-2. Bibcode: 2007NRL.....2...94A.
Other links
 |