Physics:Coulomb's constant
Coulomb's constant, the electric force constant, or the electrostatic constant (denoted ke, k or K) is a proportionality constant in electrodynamics equations. In SI units, it is exactly equal to 8987551787.3681764 N·m2·C−2, or roughly equaling 8.99×109 N·m2·C−2. It was named after the French physicist Charles-Augustin de Coulomb (1736–1806) who introduced Coulomb's law.
Value of the constant
Coulomb's constant is the constant of proportionality in Coulomb's law,
- [math]\displaystyle{ \mathbf{F} = k_\text{e}\frac{Qq}{r^2}\mathbf{\hat{e}}_r }[/math]
where êr is a unit vector in the r-direction and
- [math]\displaystyle{ k_\text{e} = \alpha \frac{\hbar c}{e^2} }[/math],
where α is the fine-structure constant, c is the speed of light, ħ is the reduced Planck constant, and e is elementary charge.[1] In SI:
- [math]\displaystyle{ k_\text{e} = \frac{1}{4\pi\varepsilon_0} }[/math],
where [math]\displaystyle{ \varepsilon_0 }[/math] is the vacuum permittivity. This formula can be derived from Gauss' law,
-
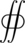 [math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = \frac{Q}{\varepsilon_0} }[/math]
[math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = \frac{Q}{\varepsilon_0} }[/math]
Taking this integral for a sphere, radius r, around a point charge, we note that the electric field points radially outwards at all times and is normal to a differential surface element on the sphere, and is constant for all points equidistant from the point charge.
-
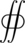 [math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = |\mathbf{E}|\mathbf{\hat{e}}_r\int_{S} dA = |\mathbf{E}|\mathbf{\hat{e}}_r \times 4\pi r^{2} }[/math]
[math]\displaystyle{ {\scriptstyle S} }[/math] [math]\displaystyle{ \mathbf{E} \cdot {\rm d}\mathbf{A} = |\mathbf{E}|\mathbf{\hat{e}}_r\int_{S} dA = |\mathbf{E}|\mathbf{\hat{e}}_r \times 4\pi r^{2} }[/math]
Noting that E = F/Q for some test charge q,
- [math]\displaystyle{ \begin{align} \mathbf{F} &= \frac{1}{4\pi\varepsilon_0}\frac{Qq}{r^2}\mathbf{\hat{e}}_r = k_\text{e}\frac{Qq}{r^2}\mathbf{\hat{e}}_r \\[8pt] \therefore k_\text{e} &= \frac{1}{4\pi\varepsilon_0} \end{align} }[/math]
In modern systems of units Coulomb's constant ke is an exact constant, in Gaussian units ke = 1, in Lorentz–Heaviside units (also called rationalized) ke = 1/4π and in SI ke = 1/4πε0, where the vacuum permittivity ε0 = 1/μ0c2 ≈ 8.85418782×10−12 F m−1, the speed of light in vacuum c is 299792458 m/s, the vacuum permeability μ0 is 4π×10−7 H m−1,[2] so that[3]
- [math]\displaystyle{ \begin{align} k_\text{e} = \frac{1}{4\pi\varepsilon_0}=\frac{c^2\mu_0}{4\pi}&=c^2\times (10^{-7}\ \mathrm{H\ m}^{-1})\\ &= 8.987\,551\,787\,368\,1764\times 10^9~\mathrm{N\ m^2\ C^{-2}}. \end{align} }[/math]
Use of Coulomb's constant
Coulomb's constant is used in many electric equations, although it is sometimes expressed as the following product of the vacuum permittivity constant:
- [math]\displaystyle{ k_\text{e} = \frac{1}{4\pi\varepsilon_0}. }[/math]
Coulomb's constant appears in many expressions including the following:
- [math]\displaystyle{ \mathbf{F}=k_\text{e}{Qq\over r^2}\mathbf{\hat{e}}_r. }[/math]
- [math]\displaystyle{ U_\text{E}(r) = k_\text{e}\frac{Qq}{r}. }[/math]
- [math]\displaystyle{ \mathbf{E} = k_\text{e} \sum_{i=1}^N \frac{Q_i}{r_i^2} \mathbf{\hat{r}}_i. }[/math]
See also
References
- ↑ Tomilin, K. (1999). "Fine-structure constant and dimension analysis". European Journal of Physics 20 (5): L39–L40. doi:10.1088/0143-0807/20/5/404. Bibcode: 1999EJPh...20L..39T.
- ↑ CODATA Value: electric constant. Physics.nist.gov. Retrieved on 2010-09-28.
- ↑ Coulomb's constant, Hyperphysics

