Physics:Halogen bond
In chemistry, a halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity.[1] Like a hydrogen bond, the result is not a formal chemical bond, but rather a strong electrostatic attraction.[2][3] Mathematically, the interaction can be decomposed in two terms: one describing an electrostatic, orbital-mixing charge-transfer and another describing electron-cloud dispersion. Halogen bonds find application in supramolecular chemistry;[2][3][4] drug design and biochemistry;[5][6] crystal engineering[6] and liquid crystals;[2] and organic catalysis.[6]
Definition
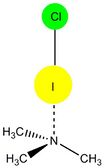
Halogen bonds occur when a halogen atom is electrostatically attracted to a partial negative charge. Necessarily, the atom must be covalently bonded in an antipodal σ-bond; the electron concentration associated with that bond leaves a positively charged "hole" on the other side.[7] Although all halogens can theoretically participate in halogen bonds, the σ-hole shrinks if the electron cloud in question polarizes poorly or the halogen is so electronegative as to polarize the associated σ-bond.[2][8] Consequently halogen-bond propensity follows the trend[9][Note 1] F < Cl < Br < I.
There is no clear distinction between halogen bonds and expanded octet partial bonds; what is superficially a halogen bond may well turn out to be a full bond in an unexpectedly relevant resonance structure.[10][11][12][13]
Donor characteristics
A halogen bond is almost collinear with the halogen atom's other, conventional bond, but the geometry of the electron-charge donor may be much more complex.
- Multi-electron donors such as ethers and amines prefer halogen bonds collinear with the lone pair and donor nucleus.
- Pyridine derivatives tend to donate halogen bonds approximately coplanar with the ring, and the two angles are about 120°.[14]
- Carbonyl, thiocarbonyl-, and selenocarbonyl groups, with a trigonal planar geometry around the Lewis donor atom, can accept one or two halogen bonds.[15]
Anions are usually better halogen-bond acceptors than neutral species: the more dissociated an ion pair is, the stronger the halogen bond formed with the anion.[16]
Comparison to other bond-like forces
A parallel relationship can easily be drawn between halogen bonding and hydrogen bonding. Both interactions revolve around an electron donor/electron acceptor relationship, between a halogen-like atom and an electron-dense one. But halogen bonding is both much stronger and more sensitive to direction than hydrogen bonding. A typical hydrogen bond has energy of formation 20 kJ/mol; known halogen bond energies range from 10–200 kJ/mol.[15]
The σ-hole concept readily extends to pnictogen, chalcogen and aerogen bonds, corresponding to atoms of Groups 15, 16 and 18 (respectively).[17]
History

In 1814, Jean-Jacques Colin discovered (to his surprise) that a mixture of dry gaseous ammonia and iodine formed a shiny, metallic-appearing liquid. Frederick Guthrie established the precise composition of the resulting I2···NH3 complex fifty years later, but the physical processes underlying the molecular interaction remained mysterious until the development of Robert S. Mulliken's theory of inner-sphere and outer-sphere interactions.[18] In Mulliken's categorization, the intermolecular interactions associated with small partial charges affect only the "inner sphere" of an atom's electron distribution; the electron redistribution associated with Lewis adducts affects the "outer sphere" instead.[19]
Then, in 1954, Odd Hassel fruitfully applied the distinction to rationalize the X-ray diffraction patterns associated with a mixture of 1,4-dioxane and bromine.[20] The patterns suggested that only 2.71 Å separated the dioxane oxygen atoms and bromine atoms, much closer than the sum (3.35 Å) of the atoms' van der Waals radii; and that the angle between the O−Br and Br−Br bond was about 180°. From these facts, Hassel concluded that halogen atoms are directly linked to electron pair donors in a direction with a bond direction that coincides with the axes of the orbitals of the lone pairs in the electron pair donor molecule.[7] For this work, Hassel was awarded the 1969 Nobel Prize in Chemistry.[21]
Dumas and coworkers first coined the term "halogen bond" in 1978, during their investigations into complexes of CCl4, CBr4, SiCl4, and SiBr4 with tetrahydrofuran, tetrahydropyran, pyridine, anisole, and di-n-butyl ether in organic solvents.[22]
However, it was not until the mid-1990s, that the nature and applications of the halogen bond began to be intensively studied. Through systematic and extensive microwave spectroscopy of gas-phase halogen bond adducts, Legon and coworkers drew attention to the similarities between halogen-bonding and better-known hydrogen-bonding interactions.[23]
In 2007, computational calculations by Politzer and Murray showed that an anisotropic electron density distribution around the halogen nucleus — the "σ-hole"[8] — underlay the high directionality of the halogen bond.[24] This hole was then experimentally observed using Kelvin probe force microscopy.[25][26]
In 2020, Kellett et al. showed that halogen bonds also have a π-covalent character similar to metal coordination bonds.[27] In August 2023 the "π-hole" was too experimentally observed[28][29]
Applications
Crystal engineering
File:Silsesquixane halogen bond.tif The strength and directionality of halogen bonds are a key tool in the discipline of crystal engineering, which attempts to shape crystal structures through close control of intermolecular interactions.[31] Halogen bonds can stabilize copolymers[32][33] or induce mesomorphism in otherwise isotropic liquids.[34] Indeed, halogen bond-induced liquid crystalline phases are known in both alkoxystilbazoles[34] and silsesquioxanes (pictured).[30] Alternatively, the steric sensitivity of halogen bonds can cause bulky molecules to crystallize into porous structures; in one notable case, halogen bonds between iodine and aromatic π-orbitals caused molecules to crystallize into a pattern that was nearly 40% void.[35]
Controlled polymerization
Conjugated polymers offer the tantalizing possibility of organic molecules with a manipulable electronic band structure, but current methods for production have an uncontrolled topology. Sun, Lauher, and Goroff discovered that certain amides ensure a linear polymerization of poly(diiododiacetylene). The underlying mechanism is a self-organization of the amides via hydrogen bonds that then transfers to the diiododiacetylene monomers via halogen bonds. Although pure diiododiacetylene crystals do not polymerize spontaneously, the halogen-bond induced organization is sufficiently strong that the cocrystals do spontaneously polymerize.[36]
-
The catalyst-monomer cocrystal. Units repeat every 5.25 Å and are oriented at 51.3˚.
-
Post-polymerization crystal structure: the oxygen atom (purple) forms a hydrogen bond (blue dashed line) and a weak halogen bond with the polymer's iodine substituents. Iodine may also form a halogen bond with the terminal nitriles (red dashed line).
Biological macromolecules

Most biological macromolecules contain few or no halogen atoms. But when molecules do contain halogens, halogen bonds are often essential to understanding molecular conformation. Computational studies suggest that known halogenated nucleobases form halogen bonds with oxygen, nitrogen, or sulfur in vitro. Interestingly, oxygen atoms typically do not attract halogens with their lone pairs, but rather the π electrons in the carbonyl or amide group.[5]
Halogen bonding can be significant in drug design as well. For example, inhibitor IDD 594 binds to human aldose reductase through a bromine halogen bond, as shown in the figure. The molecules fail to bind to each other if similar aldehyde reductase replaces the enzyme, or chlorine replaces the drug halogen, because the variant geometries inhibit the halogen bond.[37]
Notes
- ↑ Although hydrogen is sometimes considered a halogen, convention excludes hydrogen bonds from the category of halogen bonds. For a complete analysis, see § Comparison to other bond-like forces.
References
- ↑ "Definition of the Halogen Bond (IUPAC Recommendations 2013)". Pure Appl. Chem. 85 (8): 1711–1713. 2013. doi:10.1351/pac-rec-12-05-10.
- ↑ 2.0 2.1 2.2 2.3 "Halogen bonding based recognition processes: a world parallel to hydrogen bonding". Accounts of Chemical Research 38 (5): 386–395. May 2005. doi:10.1021/ar0400995. PMID 15895976.
- ↑ 3.0 3.1 "Halogen Bonding in Supramolecular Chemistry". Chemical Reviews 115 (15): 7118–7195. August 2015. doi:10.1021/cr500674c. PMID 26165273.
- ↑ "Halogen bonding: a paradigm in supramolecular chemistry". Chemistry 7 (12): 2511–2519. June 2001. doi:10.1002/1521-3765(20010618)7:12<2511::AID-CHEM25110>3.0.CO;2-T. PMID 11465442.
- ↑ 5.0 5.1 "Halogen bonds in biological molecules". Proceedings of the National Academy of Sciences of the United States of America 101 (48): 16789–16794. November 2004. doi:10.1073/pnas.0407607101. PMID 15557000. Bibcode: 2004PNAS..10116789A.
- ↑ 6.0 6.1 6.2 "The Halogen Bond". Chemical Reviews 116 (4): 2478–2601. February 2016. doi:10.1021/acs.chemrev.5b00484. PMID 26812185.
- ↑ 7.0 7.1 "Structural aspects of interatomic charge-transfer bonding". Science 170 (3957): 497–502. October 1970. doi:10.1126/science.170.3957.497. PMID 17799698. Bibcode: 1970Sci...170..497H.
- ↑ 8.0 8.1 "Halogen bonding: the sigma-hole. Proceedings of "Modeling interactions in biomolecules II", Prague, September 5th-9th, 2005". Journal of Molecular Modeling 13 (2): 291–296. February 2007. doi:10.1007/s00894-006-0130-2. PMID 16927107.
- ↑ "An overview of halogen bonding". Journal of Molecular Modeling 13 (2): 305–311. February 2007. doi:10.1007/s00894-006-0154-7. PMID 17013631.
- ↑ "Halogen Bonding versus Hydrogen Bonding: A Molecular Orbital Perspective". ChemistryOpen 1 (2): 96–105. April 2012. doi:10.1002/open.201100015. PMID 24551497.
- ↑ "DFT calculations, structural and spectroscopic studies on the products formed between IBr and N,N'-dimethylbenzoimidazole-2(3H)-thione and -2(3H)-selone". Dalton Transactions (13): 2252–2258. July 2005. doi:10.1039/B503883A. PMID 15962045.
- ↑ "Does fluorine participate in halogen bonding?". Chemistry 21 (12): 4739–4746. March 2015. doi:10.1002/chem.201405054. PMID 25652256.
- ↑ "Halogen Bonding: An Odd Chemistry?". Chemical Record 21 (5): 1252–1257. May 2021. doi:10.1002/tcr.202100060. PMID 33939244.
- ↑ "Reactions of pyridyl donors with halogens and interhalogens: an X-ray diffraction and FT-Raman investigation" (in en). Journal of Organometallic Chemistry. III Euchem Conference on Nitrogen Ligands in Organometallic Chemistry and Homogeneous Catalysis 690 (8): 1923–1934. 2005-04-15. doi:10.1016/j.jorganchem.2004.11.001. ISSN 0022-328X. https://www.sciencedirect.com/science/article/pii/S0022328X0400868X.
- ↑ 15.0 15.1 "Halogen bonding in supramolecular chemistry". Angewandte Chemie 47 (33): 6114–6127. 2008-08-04. doi:10.1002/anie.200800128. PMID 18651626.
- ↑ "Fluorous Interpenetrated Layers in a Three-Component Crystal Matrix" (in en). Crystal Growth & Design 3 (3): 355–361. 2003-05-01. doi:10.1021/cg0340244. ISSN 1528-7483. https://pubs.acs.org/doi/10.1021/cg0340244.
- ↑ "Aerogen Bonding Interaction: A New Supramolecular Force?". Angewandte Chemie 54 (25): 7340–7343. June 2015. doi:10.1002/anie.201502571. PMID 25950423.
- ↑ "Xxviii.—On the Iodide of Iodammonium". J. Chem. Soc. 16: 239–244. 1863. doi:10.1039/js8631600239. https://zenodo.org/record/1429751.
- ↑
- "Structures of Complexes Formed by Halogen Molecules with Aromatic and with Oxygenated Solvents I.". J. Am. Chem. Soc. 72 (1): 600. 1950. doi:10.1021/ja01157a151.
- "Molecular Compounds and their Spectra. II". J. Am. Chem. Soc. 74 (3): 811–824. 1952. doi:10.1021/ja01123a067.
- "Molecular Compounds and their Spectra. III. The Interaction of Electron Donors and Acceptors". J. Phys. Chem. 56 (7): 801–822. 1952. doi:10.1021/j150499a001.
- ↑ "The Structure of Bromine 1,4-Dioxanate". Acta Chem. Scand. 8: 873. 1954. doi:10.3891/acta.chem.scand.08-0873. http://actachemscand.org/pdf/acta_vol_08_p0873.pdf.
- ↑ "Structural Aspects of Interatomic Charge-Transfer Bonding". In Nobel Lectures, Chemistry 1963-1970: 314–329. 1972.
- ↑ "CX4...Base Interactions as Models of Weak Charge-transfer Interactions: Comparison with Strong Charge-transfer and Hydrogen-bond Interactions". J. Chem. Res.(S) 2: 54–57. 1978.
- ↑ "Prereactive Complexes of Dihalogens XY with Lewis Bases B in the Gas Phase: A Systematic Case for the Halogen Analogue B···XY of the Hydrogen Bond B···HX". Angewandte Chemie 38 (18): 2686–2714. September 1999. doi:10.1002/(sici)1521-3773(19990917)38:18<2686::aid-anie2686>3.0.co;2-6. PMID 10508357.
- ↑ "Halogen bonding: an electrostatically-driven highly directional noncovalent interaction". Physical Chemistry Chemical Physics 12 (28): 7748–7757. July 2010. doi:10.1039/c004189k. PMID 20571692.
- ↑ "Real-space imaging of anisotropic charge of σ-hole by means of Kelvin probe force microscopy". Science 374 (6569): 863–867. November 2021. doi:10.1126/science.abk1479. PMID 34762455.
- ↑ Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences (IOCB Prague). "First observation of an inhomogeneous electron charge distribution on an atom" (in en). https://phys.org/news/2021-11-inhomogeneous-electron-atom.html.
- ↑ "π covalency in the halogen bond". Nature Communications 11 (1): 3310. July 2020. doi:10.1038/s41467-020-17122-7. PMID 32620765.
- ↑ "Visualization of π-hole in molecules by means of Kelvin probe force microscopy". Nature Communications 14 (1): 4954. August 2023. doi:10.1038/s41467-023-40593-3. PMID 37587123.
- ↑ Institute of Organic Chemistry and Biochemistry of the CAS. "Scientists confirm decades-old theory of non-uniform distribution of electron density in aromatic molecules" (in en). https://phys.org/news/2023-08-scientists-decades-old-theory-non-uniform-electron.html.
- ↑ 30.0 30.1 "Synthesis, characterization and thermal properties of T8 type amido-POSS with p-halophenyl end-group" (in en). Journal of Organometallic Chemistry 847: 173–183. 2017-10-01. doi:10.1016/j.jorganchem.2017.05.044. ISSN 0022-328X.
- ↑ "Anion coordination and anion-templated assembly under halogen bonding control". CrystEngComm 11 (7): 1187–1196. 2009. doi:10.1039/B821300C.
- ↑ "Halogen Bonding versus Hydrogen Bonding in Driving Self-Assembly Processes Perfluorocarbon-hydrocarbon self-assembly, part IX. This work was supported by MURST (Cofinanziamento '99) and EU (COST-D12-0012).". Angewandte Chemie (Wiley-VCH) 39 (10): 1782–1786. May 2000. doi:10.1002/(SICI)1521-3773(20000515)39:10<1782::AID-ANIE1782>3.0.CO;2-5. PMID 10934360.
- ↑ "Perfluorocarbon−Hydrocarbon Self-Assembling. 1D Infinite Chain Formation Driven by Nitrogen···Iodine Interactions" (in en). Journal of the American Chemical Society 120 (32): 8261–8262. August 1998. doi:10.1021/ja9810686. ISSN 0002-7863. https://pubs.acs.org/doi/10.1021/ja9810686.
- ↑ 34.0 34.1 "Halogen bonding: a new interaction for liquid crystal formation". Journal of the American Chemical Society 126 (1): 16–17. January 2004. doi:10.1021/ja036994l. PMID 14709037.
- ↑ "Hexagonal crystalline inclusion complexes of 4-iodophenoxy trimesoate". Chemical Communications 38 (39): 4726–4728. October 2008. doi:10.1039/b809592b. PMID 18830473.
- ↑ "Preparation of poly(diiododiacetylene), an ordered conjugated polymer of carbon and iodine". Science 312 (5776): 1030–1034. May 2006. doi:10.1126/science.1124621. PMID 16709780. Bibcode: 2006Sci...312.1030S.
- ↑ 37.0 37.1 "Ultrahigh resolution drug design I: details of interactions in human aldose reductase-inhibitor complex at 0.66 A". Proteins 55 (4): 792–804. June 2004. doi:10.1002/prot.20015. PMID 15146478. "The electrostatic interaction between the Br atom of the inhibitor and the OG of Thr 113 has an unusually short distance of 2.973(4) Å. The short contact between Br and Thr 113 OG explains the selectivity of IDD 594 towards AR, because in aldehyde reductase the Thr residue is replaced by Tyr....The IDD 594-Br/Thr 113-OG interaction also contributes to the potency of the inhibitor. Other halogens, such as chlorine, cannot engage in a similar interaction (due to its lower polarizability).".
Further reading
- An early review: Bent, H. A. (1968). "Structural Chemistry of Donor-Acceptor Interactions". Chem. Rev. 68 (5): 587–648. doi:10.1021/cr60255a003.
 |


