Physics:Lorentz oscillator model
The Lorentz oscillator model (classical electron oscillator or CEO model) describes the optical response of bound charges. The model is named after the Dutch physicist Hendrik Antoon Lorentz. It is a classical, phenomenological model for materials with characteristic resonance frequencies (or other characteristic energy scales) for optical absorption, e.g. ionic and molecular vibrations, interband transitions (semiconductors), phonons, and collective excitations.[1][2]
Derivation of electron motion
The model is derived by modeling an electron orbiting a massive, stationary nucleus as a spring-mass-damper system.[2][3][4] The electron is modeled to be connected to the nucleus via a hypothetical spring and its motion is damped by via a hypothetical damper. The damping force ensures that the oscillator's response is finite at its resonance frequency. For a time-harmonic driving force which originates from the electric field, Newton's second law can be applied to the electron to obtain the motion of the electron and expressions for the dipole moment, polarization, susceptibility, and dielectric function.[4]
Equation of motion for electron oscillator:
where
- is the displacement of charge from the rest position,
- is time,
- is the relaxation time/scattering time,
- is a constant factor characteristic of the spring,
- is the effective mass of the electron,
- is the resonance frequency of the oscillator,
- is the elementary charge,
- is the electric field.
For time-harmonic fields:
The stationary solution of this equation of motion is:
The fact that the above solution is complex means there is a time delay (phase shift) between the driving electric field and the response of the electron's motion.[4]
Dipole moment
The displacement, , induces a dipole moment, , given by
is the polarizability of single oscillator, given by
Three distinct scattering regimes can be interpreted corresponding to the dominant denominator term in the dipole moment:[5]
| Regime | Condition | Dispersion Scaling | Phase Shift |
|---|---|---|---|
| Thomson scattering | 0° | ||
| Shneider-Miles scattering | 90° | ||
| Rayleigh scattering | 180° |
Polarization
The polarization is the dipole moment per unit volume. For macroscopic material properties N is the density of charges (electrons) per unit volume. Considering that each electron is acting with the same dipole moment we have the polarization as below
Electric displacement
The electric displacement is related to the polarization density by
Dielectric function
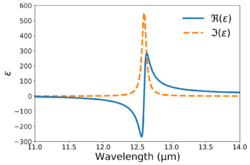
The complex dielectric function is given the following (in Gaussian units): where and is the so-called plasma frequency.
In practice, the model is commonly modified to account for multiple absorption mechanisms present in a medium. The modified version is given by[7] where and
- is the value of the dielectric function at infinite frequency, which can be used as an adjustable parameter to account for high frequency absorption mechanisms,
- and is related to the strength of the th absorption mechanism,
- .
Separating the real and imaginary components,
Complex conductivity
The complex optical conductivity in general is related to the complex dielectric function (in Gaussian units) as
Substituting the formula of in the equation above we obtain
Separating the real and imaginary components,
See also
- Cauchy equation
- Sellmeier equation
- Forouhi–Bloomer model
- Tauc–Lorentz model
- Brendel–Bormann oscillator model
References
- ↑ Lorentz, Hendrik Antoon (1909) (in en). The theory of electrons and its applications to the phenomena of light and radiant heat. Bd. XXIX;Bd. 29. New York; Leipzig: B.G. Teubner. OCLC 535812.
- ↑ 2.0 2.1 Dressel, Martin; Grüner, George (2002). "Semiconductors" (in en). Electrodynamics of Solids: Optical Properties of Electrons in Matter. Cambridge. pp. 136–172. doi:10.1017/CBO9780511606168.008. ISBN 9780521592536.
- ↑ Almog, I. F.; Bradley, M. S.; Bulovic, V. (2011). "The Lorentz Oscillator and its Applications". Massachusetts Institute of Technology. https://ocw.mit.edu/courses/electrical-engineering-and-computer-science/6-007-electromagnetic-energy-from-motors-to-lasers-spring-2011/readings/MIT6_007S11_lorentz.pdf.
- ↑ 4.0 4.1 4.2 Colton, John (2020). "Lorentz Oscillator Model". Brigham Young University. https://physics.byu.edu/faculty/colton/docs/phy442-resources/Lorentz-oscillator-model.pdf.
- ↑ Patel, Adam (2021). "Thomson and collisional regimes of in-phase coherent microwave scattering off gaseous microplasmas". Scientific Reports 11 (1): 23389. doi:10.1038/s41598-021-02500-y. PMID 34862396. Bibcode: 2021NatSR..1123389P.
- ↑ Spitzer, W. G.; Kleinman, D.; Walsh, D. (1959). "Infrared Properties of Hexagonal Silicon Carbide". Physical Review 113 (1): 127–132. doi:10.1103/PhysRev.113.127. Bibcode: 1959PhRv..113..127S. https://doi.org/10.1103/PhysRev.113.127. Retrieved 2021-11-24.
- ↑ Zhang, Z. M.; Lefever-Button, G.; Powell, F. R. (1998). "Infrared Refractive Index and Extinction Coefficient of Polyimide Films". International Journal of Thermophysics 19 (3): 905–916. doi:10.1023/A:1022655309574. https://doi.org/10.1023/A:1022655309574. Retrieved 2021-11-24.
 |
