Physics:Polyhydride
A polyhydride or superhydride is a compound that contains an abnormally large amount of hydrogen. This can be described as high hydrogen stoichiometry. Examples include iron pentahydride FeH
5, LiH
6, and LiH
7. By contrast, the more well known lithium hydride only has one hydrogen atom.[1]
Polyhydrides are only known to be stable under high pressure.[1]
Polyhydrides are important because they can form substances with a very high density of hydrogen. They may resemble the elusive metallic hydrogen, but can be made under lower pressures. One possibility is that they could be superconductors. Hydrogen sulfide under high pressures forms SH
3 units, and can be a superconductor at 203 K (−70 °C) and a pressure of 1.5 million atmospheres.[1]
Structures
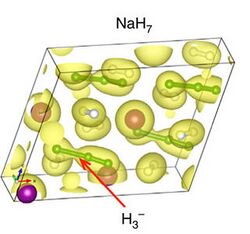
7, which contains H−
3 complexes. The coloured balls in the isosurface, plotted at the level of 0.07 electrons*Å−3. One of H
2 molecules is bonded to a hydrogen atom in the NaH unit with a bond length of 1.25 Å, forming a H−
3 linear anion.
The polyhydrides of alkaline earth and alkali metals contain cage structures. Also hydrogen may be clustered into H−
, H−
3, or H
2 units. Polyhydrides of transition metals may have the hydrogen atoms arranged around the metal atom. Computations suggest that increasing hydrogen levels will reduce the dimensionality of the metal arrangement, so that layers form separated by hydrogen sheets.[1] The H−
3 substructure is linear.[2]
H+
3 would form triangular structures in the hypothetical H
5Cl.[2]
Compounds
When sodium hydride is compressed with hydrogen, NaH
3 and NaH
7 form. These are formed at 30 GPa and 2,100 K.[2]
Heating and compressing a metal with ammonia borane avoids using bulky hydrogen, and produces boron nitride as a decomposition product in addition to the polyhydride.[3]
| formula | name | temperature
°C |
pressure
GPa |
crystal structure | space group | a Å | b | c | β | cell volume | formulae
per unit cell |
Tc K | Comment | refs |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LiH 2 |
lithium dihydride | 27 | 130 | [4] | ||||||||||
| LiH 6 |
Lithium hexahydride | [1] | ||||||||||||
| LiH 7 |
Lithium heptahydride | [1] | ||||||||||||
| NaH 3 |
sodium trihydride | orthorhombic | Cmcm | 3.332 Å | 6.354 Å | 4.142 Å | 90 | 87.69 | 4 | [2] | ||||
| NaH 7 |
sodium heptahydride | monoclinic | Cc | 6.99 | 3.597 | 5.541 | 69.465 | 130.5 | [2] | |||||
| CaH x |
500 | 22 | double hexagon | [5] | ||||||||||
| CaH x |
600 | 121 | [5] | |||||||||||
| SrH 6 |
pseudo cubic | Pm3m | semiconductor
metallize > 220 GPa |
[6] | ||||||||||
| Sr 3H 13 |
C2/m | [6] | ||||||||||||
| SrH 22 |
138 | triclinic | P1 | [6] | ||||||||||
| BaH 12 |
Barium dodecahydride | 75 | pseudo cubic | 5.43 | 5.41 | 5.37 | 39.48 | 20K | [7][8] | |||||
| FeH 5 |
iron pentahydride | 1200 | 66 | tetragonal | I4/mmm | [1] | ||||||||
| H 3S |
Sulfur trihydride | 25 | 150 | cubic | Im3m | 203K | [9] | |||||||
| H 3Se |
Selenium trihydride | 10 | [10] | |||||||||||
| YH 4 |
yttrium tetrahydride | 700 | 160 | I4/mmm | [11] | |||||||||
| YH 6 |
yttrium hexahydride | 700 | 160 | Im-3m | 224 | [11][12][13] | ||||||||
| YH 9 |
yttrium nonahydride | 400 | 237 | P63/mmc | 243 | [11] | ||||||||
| LaH 10 |
Lanthanum decahydride | 1000 | 170 | cubic | Fm3m | 5.09 | 5.09 | 5.09 | 132 | 4 | 250K | [14][15] | ||
| LaH 10 |
Lanthanum decahydride | 25 | 121 | Hexagonal | R3m | 3.67 | 3.67 | 8.83 | 1 | [14] | ||||
| LaD 11 |
Lanthanum undecahydride | 2150 | 130-160 | Tetragonal | P4/nmm | 168 | [15] | |||||||
| LaH 12 |
Lanthanum dodecahydride | Cubic | insulating | [15] | ||||||||||
| LaH 7 |
Lanthanum heptahydride | 25 | 109 | monoclinic | C2/m | 6.44 | 3.8 | 3.69 | 135 | 63.9 | 2 | [14] | ||
| CeH 9 |
Cerium nonahydride | 93 | hexagonal | P63/mmc | 3.711 | 5.543 | 33.053 | 100K | [16] | |||||
| CeH 10 |
Cerium decahydride | Fm3m | 115K | [17] | ||||||||||
| PrH 9 |
Praseodymium nonahydride | 90-140 | P63/mmc | 3.60 | 5.47 | 61.5 | 55K 9K | [18][19] | ||||||
| PrH 9 |
Praseodymium nonahydride | 120 | F43m | 4.98 | 124 | 69K | [18] | |||||||
| NdH 4 |
Neodymium tetrahydride | 85-135 | tetragonal | I4/mmm | 2.8234 | 5,7808 | [20] | |||||||
| NdH 7 |
Neodymium heptahydride | 85-135 | monoclinic | C2/c | 3.3177 | 6.252 | 5.707 | 89.354 | [20] | |||||
| NdH 9 |
Neodymium nonahydride | 110-130 | hexagonal | P63/mmc | 3.458 | 5.935 | [20] | |||||||
| EuH 4 |
50-130 | I4/mmm | [21] | |||||||||||
| Eu 8H 46 |
1600 | 130 | cubic | Pm3n | 5.865 | [21] | ||||||||
| EuH 9 |
Europium nonahydride | 86-130 | cubic | F43m | [21] | |||||||||
| EuH 9 |
Europium nonahydride | >130 | hexagonal | P63/mmc | [21] | |||||||||
| ThH 4 |
Thorium tetrahydride | 86 | I4/mmm | 2.903 | 4.421 | 57.23 | 2 | [3] | ||||||
| ThH 4 |
Thorium tetrahydride | 88 | trigonal | P321 | 5.500 | 3.29 | 86.18 | [3] | ||||||
| ThH 4 |
Thorium tetrahydride | orthorhombic | Fmmm | [3] | ||||||||||
| ThH 6 |
Thorium hexahydride | 86-104 | Cmc21 | 32.36 | [3] | |||||||||
| ThH 9 |
Thorium nonahydride | 2100 | 152 | hexagonal | P63/mmc | 3.713 | 5.541 | 66.20 | [3] | |||||
| ThH 10 |
Thorium decahydride | 1800 | 85-185 | cubic | Fm3m | 5.29 | 148.0 | 161 | [3] | |||||
| ThH 10 |
Thorium decahydride | <85 | Immm | 5.304 | 3.287 | 3.647 | 74.03 | [3] | ||||||
| UH 7 |
Uranium heptahydride | 2000 | 63 | fcc | P63/mmc | [22] | ||||||||
| UH 8 |
Uranium octahydride | 300 | 1-55 | fcc | Fm3m | [22] | ||||||||
| UH 9 |
Uranium nonahydride | 40-55 | fcc | P63/mmc | [22] |
Predicted
Using computational chemistry many other polyhydrides are predicted, including LiH
8,[23]
LiH
9,[24] LiH
10,[24] CsH
3,[25] KH
5, RbH
5,[26] RbH
9,[23] NaH
9, BaH
6,[26] CaH
6,[27] MgH
4, MgH
12, MgH
16,[28] SrH
4,[29] SrH
10, SrH
12,[23] ScH
4, ScH
6, ScH
8,[30] YH
4 and YH
6,[31] YH
24, LaH
8, LaH
10,[32] YH
9, LaH
11, CeH
8, CeH
9, CeH
10,[33] PrH
8, PrH
9,[34] ThH
6, ThH
7 and ThH
10,[35] U
2H
13, UH
7, UH
8, UH
9,[22] AlH
5,[36] GaH
5, InH
5,[23] SnH
8, SnH
12, SnH
14,[37] PbH
8,[38] SiH
8 (subsequently discovered),[23] GeH
8,[39] (although Ge
3H
11 may be stable instead)[40] AsH
8, SbH
4,[41] BiH
4, BiH
5, BiH
6,[42] H
3Se,[43] H
3S,[44] Te
2H
5, TeH
4,[45] PoH
4, PoH
6,[23] H
2F, H
3F,[23] H
2Cl, H
3Cl, H
5Cl, H
7Cl,[46] H
2Br, H
3Br, H
4Br, H
5Br, H
5I,[23] XeH
2, XeH
4.[47]
Among the transition elements, VH
8 in a C2/m structure around 200 GPa is predicted to have a superconducting transition temperature of 71.4 K. VH
5 in a P63/mmm space group has a lower transition temperature.[48]
Properties
Superconduction
Under suitably high pressures polyhydrides may become superconducting. Characteristics of substances that are predicted to have high superconducting temperatures are a high phonon frequency, which will happen for light elements, and strong bonds. Hydrogen is the lightest and so will have the highest frequency of vibration. Even changing the isotope to deuterium will lower the frequency and lower the transition temperature. Compounds with more hydrogen will resemble the predicted metallic hydrogen. However, superconductors also tend to be substances with high symmetry and also need the electrons not to be locked into molecular subunits, and require large numbers of electrons in states near the Fermi level. There should also be electron-phonon coupling which happens when the electric properties are tied to the mechanical position of the hydrogen atoms.[34][49][50] The highest superconduction critical temperatures are predicted to be in groups 3 and 3 of the periodic table. Late transitions elements, heavy lanthanides or actinides have extra d- or f-electrons that interfere with superconductivity.[51]
For example, lithium hexahydride is predicted to lose all electrical resistance below 38 K at a pressure of 150 GPa. The hypothetical LiH
8 has a predicted superconducting transition temperature at 31 K at 200 GPa.[52] MgH
6 is predicted to have a Tc of 400 K around 300 GPa.[53] CaH
6 could have a Tc of 260 K at 120 GPa. PH
3 doped H
3S is also predicted to have a transition temperature above the 203 K measured for H
3S (contaminated with solid sulfur).[54] Rare earth and actinide polyhydrides may also have highish transition temperatures, for example, ThH
10 with Tc = 241 K.[35] UH
8, which can be decompressed to room temperature without decomposition, is predicted to have a transition temperature of 193 K.[35] AcH
10, if it could be ever made, is predicted to superconduct at temperatures over 204 K, and AcH
10 would be similarly conducting under lower pressures (150 GPa).[55]
H
3Se actually is a van der Waals solid with formula 2H
2Se · H
2 with a measured Tc of 105 K under a pressure of 135 GPa.[10]
Ternary superhydrides
Ternary superhydrides open up the possibility of many more formulas.[56] For example, Li
2MgH
16 may also be superconducting at high temperatures (200 °C).[57] A compound of lanthanum, boron and hydrogen is speculated to be a "hot" superconductor (550 K).[58][59] Elements may substitute for others and so modify the properties eg (La,Y)H
6 and (La,Y)H
10 can be made to have a slightly higher critical temperature than YH
6 or LaH
10.[60]
See also
- Potassium nonahydridorhenate, stable at ordinary pressures
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Pépin, C. M.; Geneste, G.; Dewaele, A.; Mezouar, M.; Loubeyre, P. (27 July 2017). "Synthesis of FeH5 : A layered structure with atomic hydrogen slabs". Science 357 (6349): 382–385. doi:10.1126/science.aan0961. PMID 28751605. Bibcode: 2017Sci...357..382P.
- ↑ 2.0 2.1 2.2 2.3 2.4 Struzhkin, Viktor V.; Kim, Duck Young; Stavrou, Elissaios; Muramatsu, Takaki; Mao, Ho-kwang; Pickard, Chris J.; Needs, Richard J.; Prakapenka, Vitali B. et al. (28 July 2016). "Synthesis of sodium polyhydrides at high pressures". Nature Communications 7: 12267. doi:10.1038/ncomms12267. PMID 27464650. Bibcode: 2016NatCo...712267S.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 Semenok, D. V.; Kvashnin, A. G; Ivanova, A. G.; Troayn, I. A.; Oganov, A. R. (2019). Synthesis of ThH4, ThH6, ThH9 and ThH10 : a route to room-temperature superconductivity. doi:10.13140/RG.2.2.31274.88003. https://www.researchgate.net/publication/331354024.
- ↑ Pépin, Charles; Loubeyre, Paul; Occelli, Florent; Dumas, Paul (23 June 2015). "Synthesis of lithium polyhydrides above 130 GPa at 300 K". Proceedings of the National Academy of Sciences 112 (25): 7673–7676. doi:10.1073/pnas.1507508112. PMID 26056306. Bibcode: 2015PNAS..112.7673P.
- ↑ 5.0 5.1 Mishra, Ajay Kumar; Ahart, Muhtar; Somayazulu, Maddury; Park, C. Y; Hemley, Russel J (2017-03-13). "Synthesis of Calcium polyhydrides at high pressure and high temperature". Bulletin of the American Physical Society 62 (4): B35.008. Bibcode: 2017APS..MARB35008M. https://meetings.aps.org/Meeting/MAR17/Session/B35.8.
- ↑ 6.0 6.1 6.2 Semenok, Dmitrii V.; Chen, Wuhao; Huang, Xiaoli; Zhou, Di; Kruglov, Ivan A.; Mazitov, Arslan B.; Galasso, Michele; Tantardini, Christian et al. (2022-06-03). "Sr‐Doped Superionic Hydrogen Glass: Synthesis and Properties of SrH 22" (in en). Advanced Materials 34 (27): 2200924. doi:10.1002/adma.202200924. ISSN 0935-9648. PMID 35451134. Bibcode: 2022AdM....3400924S. https://onlinelibrary.wiley.com/doi/10.1002/adma.202200924.
- ↑ chen, Wuhao (April 2020). "High-Pressure Synthesis of Barium Superhydrides: Pseudocubic BaH12" (in en). https://www.researchgate.net/publication/340930295.
- ↑ Chen, Wuhao; Semenok, Dmitrii V.; Kvashnin, Alexander G.; Huang, Xiaoli; Kruglov, Ivan A.; Galasso, Michele; Song, Hao; Duan, Defang et al. (December 2021). "Synthesis of molecular metallic barium superhydride: pseudocubic BaH12". Nature Communications 12 (1): 273. doi:10.1038/s41467-020-20103-5. PMID 33431840. Bibcode: 2021NatCo..12..273C.
- ↑ Shylin, S. I.; Ksenofontov, V.; Troyan, I. A.; Eremets, M. I.; Drozdov, A. P. (September 2015). "Conventional superconductivity at 203 kelvin at high pressures in the sulfur hydride system" (in en). Nature 525 (7567): 73–76. doi:10.1038/nature14964. ISSN 1476-4687. PMID 26280333. Bibcode: 2015Natur.525...73D.
- ↑ 10.0 10.1 Mishra, A. K.; Somayazulu, M.; Ahart, M.; Karandikar, A.; Hemley, R. J.; Struzhkin, V. (9 March 2018). "Novel Synthesis Route and Observation of Superconductivity in the Se-H System at Extreme Conditions". APS March Meeting Abstracts 63 (1): X38.008. Bibcode: 2018APS..MARX38008M. http://meetings.aps.org/Meeting/MAR18/Session/X38.8.
- ↑ 11.0 11.1 11.2 Kong, P. P.; Minkov, V. S.; Kuzovnikov, M. A.; Besedin, S. P.; Drozdov, A. P.; Mozaffari, S.; Balicas, L.; Balakirev, F. F.; Prakapenka, V. B.; Greenberg, E.; Knyazev, D. A. (2019-09-23). "Superconductivity up to 243 K in yttrium hydrides under high pressure". arXiv:1909.10482 [cond-mat.supr-con].
- ↑ Troyan, I. A.; Semenok, D. V.; Kvashnin, A. G.; Ivanova, A. G.; Prakapenka, V. B.; Greenberg, E.; Gavriliuk, A. G.; Lyubutin, I. S. et al. (2021). "Anomalous High‐Temperature Superconductivity in YH 6" (in en). Advanced Materials 33 (15): e2006832. doi:10.1002/adma.202006832. ISSN 0935-9648. PMID 33751670. Bibcode: 2021AdM....3306832T.
- ↑ Troyan, Ivan A.; Semenok, Dmitrii V.; Kvashnin, Alexander G.; Sadakov, Andrey V.; Sobolevskiy, Oleg A.; Pudalov, Vladimir M.; Ivanova, Anna G.; Prakapenka, Vitali B. et al. (10 March 2021). "Anomalous High‐Temperature Superconductivity in YH 6". Advanced Materials 33 (15): 2006832. doi:10.1002/adma.202006832. ISSN 0935-9648. PMID 33751670. Bibcode: 2021AdM....3306832T.
- ↑ 14.0 14.1 14.2 Geballe, Zachary M.; Liu, Hanyu; Mishra, Ajay K.; Ahart, Muhtar; Somayazulu, Maddury; Meng, Yue; Baldini, Maria; Hemley, Russell J. (15 January 2018). "Synthesis and Stability of Lanthanum Superhydrides". Angewandte Chemie International Edition 57 (3): 688–692. doi:10.1002/anie.201709970. PMID 29193506. Bibcode: 2018APS..MARX38010G.
- ↑ 15.0 15.1 15.2 Drozdov, A. P.; Kong, P. P.; Minkov, V. S.; Besedin, S. P.; Kuzovnikov, M. A.; Mozaffari, S.; Balicas, L.; Balakirev, F. F. et al. (22 May 2019). "Superconductivity at 250 K in lanthanum hydride under high pressures". Nature 569 (7757): 528–531. doi:10.1038/s41586-019-1201-8. PMID 31118520. Bibcode: 2019Natur.569..528D.
- ↑ Salke, Nilesh P. (May 2018). "Synthesis of clathrate cerium superhydride CeH9 below 100 GPa with atomic hydrogen sublattice". Nature Communications 10 (1): 4453. doi:10.1038/s41467-019-12326-y. PMID 31575861.
- ↑ Chen, Wuhao; Semenok, Dmitrii V.; Huang, Xiaoli; Shu, Haiyun; Li, Xin; Duan, Defang; Cui, Tian; Oganov, Artem R. (2021-09-09). "High-Temperature Superconducting Phases in Cerium Superhydride with a T c up to 115 K below a Pressure of 1 Megabar" (in en). Physical Review Letters 127 (11): 117001. doi:10.1103/PhysRevLett.127.117001. ISSN 0031-9007. PMID 34558917. Bibcode: 2021PhRvL.127k7001C. https://link.aps.org/doi/10.1103/PhysRevLett.127.117001.
- ↑ 18.0 18.1 Zhou, Di; Semenok, Dmitrii; Defang Duan; Xie, Hui; Xiaoli Huang; Wuhao Chen; Li, Xin; Bingbing Liu et al. (2019). "Superconducting Praseodymium Superhydrides" (in en). Unpublished 6 (9): eaax6849. doi:10.1126/sciadv.aax6849. PMID 32158937. Bibcode: 2020SciA....6.6849Z.
- ↑ Zhou, Di; Semenok, Dmitrii V.; Duan, Defang; Xie, Hui; Chen, Wuhao; Huang, Xiaoli; Li, Xin; Liu, Bingbing et al. (February 2020). "Superconducting praseodymium superhydrides" (in en). Science Advances 6 (9): eaax6849. doi:10.1126/sciadv.aax6849. ISSN 2375-2548. PMID 32158937. Bibcode: 2020SciA....6.6849Z.
- ↑ 20.0 20.1 20.2 Zhou, Di; Semenok, Dmitrii V.; Xie, Hui; Huang, Xiaoli; Duan, Defang; Aperis, Alex; Oppeneer, Peter M.; Galasso, Michele et al. (2020-02-12). "High-Pressure Synthesis of Magnetic Neodymium Polyhydrides". Journal of the American Chemical Society 142 (6): 2803–2811. doi:10.1021/jacs.9b10439. ISSN 0002-7863. PMID 31967807. https://doi.org/10.1021/jacs.9b10439.
- ↑ 21.0 21.1 21.2 21.3 Semenok, Dmitrii V.; Zhou, Di; Kvashnin, Alexander G.; Huang, Xiaoli; Galasso, Michele; Kruglov, Ivan A.; Ivanova, Anna G.; Gavriliuk, Alexander G. et al. (2020-12-09). "Novel Strongly Correlated Europium Superhydrides" (in en). The Journal of Physical Chemistry Letters 12 (1): 32–40. doi:10.1021/acs.jpclett.0c03331. ISSN 1948-7185. PMID 33296213. https://pubs.acs.org/doi/10.1021/acs.jpclett.0c03331.
- ↑ 22.0 22.1 22.2 22.3 Kruglov, Ivan A.; Kvashnin, Alexander G.; Goncharov, Alexander F.; Oganov, Artem R.; Lobanov, Sergey; Holtgrewe, Nicholas; Yanilkin, Alexey V. (17 August 2017). "High-temperature superconductivity of uranium hydrides at near-ambient conditions". arXiv:1708.05251 [cond-mat.mtrl-sci].
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 Duan, Defang; Liu, Yunxian; Ma, Yanbin; Shao, Ziji; Liu, Bingbing; Cui, Tian (28 April 2016). "Structure and superconductivity of hydrides at high pressures". National Science Review 4: 121–135. doi:10.1093/nsr/nww029.
- ↑ 24.0 24.1 Chen, Yangmei; Geng, Hua Y.; Yan, Xiaozhen; Sun, Yi; Wu, Qiang; Chen, Xiangrong (2017). "Prediction of Stable Ground-State Lithium Polyhydrides under High Pressures". Inorganic Chemistry 56 (7): 3867–3874. doi:10.1021/acs.inorgchem.6b02709. PMID 28318270.
- ↑ Shamp, Andrew; Hooper, James; Zurek, Eva (3 September 2012). "Compressed Cesium Polyhydrides: Cs+ Sublattices and H3- Three-Connected Nets". Inorganic Chemistry 51 (17): 9333–9342. doi:10.1021/ic301045v. PMID 22897718.
- ↑ 26.0 26.1 Zurek, Eva (6 June 2016). "Hydrides of the Alkali Metals and Alkaline Earth Metals Under Pressure". Comments on Inorganic Chemistry 37 (2): 78–98. doi:10.1080/02603594.2016.1196679.
- ↑ Wang, H.; Tse, J. S.; Tanaka, K.; Iitaka, T.; Ma, Y. (6 April 2012). "Superconductive sodalite-like clathrate calcium hydride at high pressures". Proceedings of the National Academy of Sciences 109 (17): 6463–6466. doi:10.1073/pnas.1118168109. PMID 22492976. Bibcode: 2012PNAS..109.6463W.
- ↑ Lonie, David C.; Hooper, James; Altintas, Bahadir; Zurek, Eva (19 February 2013). "Metallization of magnesium polyhydrides under pressure". Physical Review B 87 (5): 054107. doi:10.1103/PhysRevB.87.054107. Bibcode: 2013PhRvB..87e4107L.
- ↑ Hooper, James; Terpstra, Tyson; Shamp, Andrew; Zurek, Eva (27 March 2014). "Composition and Constitution of Compressed Strontium Polyhydrides". The Journal of Physical Chemistry C 118 (12): 6433–6447. doi:10.1021/jp4125342.
- ↑ Qian, Shifeng (2017). "Theoretical study of stability and superconductivity of". Physical Review B 96 (9): 094513. doi:10.1103/physrevb.96.094513. Bibcode: 2017PhRvB..96i4513Q.
- ↑ Li, Yinwei; Hao, Jian; Liu, Hanyu; Tse, John S.; Wang, Yanchao; Ma, Yanming (5 May 2015). "Pressure-stabilized superconductive yttrium hydrides". Scientific Reports 5 (1): 9948. doi:10.1038/srep09948. PMID 25942452. Bibcode: 2015NatSR...5E9948L.
- ↑ Liu, Hanyu; Naumov, Ivan I.; Hoffmann, Roald; Ashcroft, N. W.; Hemley, Russell J. (3 July 2017). "Potential high-Tc superconducting lanthanum and yttrium hydrides at high pressure". Proceedings of the National Academy of Sciences 114 (27): 6990–6995. doi:10.1073/pnas.1704505114. PMID 28630301. Bibcode: 2017PNAS..114.6990L.
- ↑ Tsuppayakorn-aek, Prutthipong; Pinsook, Udomsilp; Luo, Wei; Ahuja, Rajeev; Bovornratanaraks, Thiti (12 August 2020). "Superconductivity of Superhydride CeH10 under High Pressure". Materials Research Express 7 (8): 086001. doi:10.1088/2053-1591/ababc2. Bibcode: 2020MRE.....7h6001T. https://iopscience.iop.org/article/10.1088/2053-1591/ababc2/meta.
- ↑ 34.0 34.1 Peng, Feng; Sun, Ying; Pickard, Chris J.; Needs, Richard J.; Wu, Qiang; Ma, Yanming (8 September 2017). "Hydrogen Clathrate Structures in Rare Earth Hydrides at High Pressures: Possible Route to Room-Temperature Superconductivity". Physical Review Letters 119 (10): 107001. doi:10.1103/PhysRevLett.119.107001. PMID 28949166. Bibcode: 2017PhRvL.119j7001P. https://www.repository.cam.ac.uk/bitstream/handle/1810/267416/RE-H%20compound.pdf?sequence=1.
- ↑ 35.0 35.1 35.2 Kvashnin, Alexander G.; Semenok, Dmitry V.; Kruglov, Ivan A.; Oganov, Artem R. (November 2017). "High-Temperature Superconductivity in Th-H System at Pressure Conditions". doi:10.1021/acsami.8b17100.
- ↑ Hou, Pugeng; Zhao, Xiusong; Tian, Fubo; Li, Da; Duan, Defang; Zhao, Zhonglong; Chu, Binhua; Liu, Bingbing et al. (2015). "High pressure structures and superconductivity of AlH3(H2) predicted by first principles". RSC Adv. 5 (7): 5096–5101. doi:10.1039/C4RA14990D. Bibcode: 2015RSCAd...5.5096H.
- ↑ Mahdi Davari Esfahani, M.; Wang, Zhenhai; Oganov, Artem R.; Dong, Huafeng; Zhu, Qiang; Wang, Shengnan; Rakitin, Maksim S.; Zhou, Xiang-Feng (11 March 2016). "Superconductivity of novel tin hydrides (Snn Hm) under pressure". Scientific Reports 6 (1): 22873. doi:10.1038/srep22873. PMID 26964636. Bibcode: 2016NatSR...622873M.
- ↑ Cheng, Ya; Zhang, Chao; Wang, Tingting; Zhong, Guohua; Yang, Chunlei; Chen, Xiao-Jia; Lin, Hai-Qing (12 November 2015). "Pressure-induced superconductivity in H2-containing hydride PbH4(H2)2". Scientific Reports 5 (1): 16475. doi:10.1038/srep16475. PMID 26559369. Bibcode: 2015NatSR...516475C.
- ↑ Szcze¸śniak, R.; Szcze¸śniak, D.; Durajski, A.P. (April 2014). "Thermodynamics of the superconducting phase in compressed GeH4(H2)2". Solid State Communications 184: 6–11. doi:10.1016/j.ssc.2013.12.036. Bibcode: 2014SSCom.184....6S.
- ↑ Davari Esfahani, M. Mahdi; Oganov, Artem R.; Niu, Haiyang; Zhang, Jin (10 April 2017). "Superconductivity and unexpected chemistry of germanium hydrides under pressure". Physical Review B 95 (13): 134506. doi:10.1103/PhysRevB.95.134506. Bibcode: 2017PhRvB..95m4506D.
- ↑ Fu, Yuhao; Du, Xiangpo; Zhang, Lijun; Peng, Feng; Zhang, Miao; Pickard, Chris J.; Needs, Richard J.; Singh, David J. et al. (22 March 2016). "High-Pressure Phase Stability and Superconductivity of Pnictogen Hydrides and Chemical Trends for Compressed Hydrides". Chemistry of Materials 28 (6): 1746–1755. doi:10.1021/acs.chemmater.5b04638.
- ↑ Ma, Yanbin; Duan, Defang; Li, Da; Liu, Yunxian; Tian, Fubo; Yu, Hongyu; Xu, Chunhong; Shao, Ziji; Liu, Bingbing; Cui, Tian (17 November 2015). "High-pressure structures and superconductivity of bismuth hydrides". arXiv:1511.05291 [cond-mat.supr-con].
- ↑ Zhang, Shoutao; Wang, Yanchao; Zhang, Jurong; Liu, Hanyu; Zhong, Xin; Song, Hai-Feng; Yang, Guochun; Zhang, Lijun et al. (22 October 2015). "Phase Diagram and High-Temperature Superconductivity of Compressed Selenium Hydrides". Scientific Reports 5 (1): 15433. doi:10.1038/srep15433. PMID 26490223. Bibcode: 2015NatSR...515433Z.
- ↑ Durajski, Artur P.; Szczęśniak, Radosław (30 June 2017). "First-principles study of superconducting hydrogen sulfide at pressure up to 500 GPa". Scientific Reports 7 (1): 4473. doi:10.1038/s41598-017-04714-5. PMID 28667259. Bibcode: 2017NatSR...7.4473D.
- ↑ Zhong, Xin; Wang, Hui; Zhang, Jurong; Liu, Hanyu; Zhang, Shoutao; Song, Hai-Feng; Yang, Guochun; Zhang, Lijun et al. (4 February 2016). "Tellurium Hydrides at High Pressures: High-Temperature Superconductors". Physical Review Letters 116 (5): 057002. doi:10.1103/PhysRevLett.116.057002. PMID 26894729. Bibcode: 2016PhRvL.116e7002Z.
- ↑ Duan, Defang; Huang, Xiaoli; Tian, Fubo; Liu, Yunxian; Li, Da; Yu, Hongyu; Liu, Bingbing; Tian, Wenjing et al. (12 November 2015). "Predicted Formation of H3+ in Solid Halogen Polyhydrides at High Pressures". The Journal of Physical Chemistry A 119 (45): 11059–11065. doi:10.1021/acs.jpca.5b08183. PMID 26469181. Bibcode: 2015JPCA..11911059D.
- ↑ Yan, Xiaozhen; Chen, Yangmei; Kuang, Xiaoyu; Xiang, Shikai (28 September 2015). "Structure, stability, and superconductivity of new Xe–H compounds under high pressure". The Journal of Chemical Physics 143 (12): 124310. doi:10.1063/1.4931931. PMID 26429014. Bibcode: 2015JChPh.143l4310Y.
- ↑ Li, Xiaofeng; Peng, Feng (2 November 2017). "Superconductivity of Pressure-Stabilized Vanadium Hydrides". Inorganic Chemistry 56 (22): 13759–13765. doi:10.1021/acs.inorgchem.7b01686. PMID 29094931.
- ↑ Pietronero, Luciano; Boeri, Lilia; Cappelluti, Emmanuele; Ortenzi, Luciano (9 September 2017). "Conventional/unconventional superconductivity in high-pressure hydrides and beyond: insights from theory and perspectives". Quantum Studies: Mathematics and Foundations 5: 5–21. doi:10.1007/s40509-017-0128-8.
- ↑ Pinsook, Udomsilp (July 2020). "In search for near-room-temperature superconducting critical temperature of metal superhydrides under high pressure: A review". Journal of Metals, Materials and Minerals 30: 31. doi:10.14456/jmmm.2020.18. http://jmmm.material.chula.ac.th/index.php/jmmm/article/view/858.
- ↑ Semenok, Dmitrii V.; Kruglov, Ivan A.; Savkin, Igor A.; Kvashnin, Alexander G.; Oganov, Artem R. (April 2020). "On Distribution of Superconductivity in Metal Hydrides". Current Opinion in Solid State and Materials Science 24 (2): 100808. doi:10.1016/j.cossms.2020.100808. Bibcode: 2020COSSM..24j0808S.
- ↑ Xie, Yu; Li, Quan; Oganov, Artem R.; Wang, Hui (31 January 2014). "Superconductivity of lithium-doped hydrogen under high pressure". Acta Crystallographica Section C 70 (2): 104–111. doi:10.1107/S2053229613028337. PMID 24508954.
- ↑ Szczȩśniak, R.; Durajski, A. P. (13 July 2016). "Superconductivity well above room temperature in compressed MgH6". Frontiers of Physics 11 (6): 117406. doi:10.1007/s11467-016-0578-1. Bibcode: 2016FrPhy..11k7406S.
- ↑ Eremets, M I; Drozdov, A P (30 November 2016). "High-temperature conventional superconductivity". Physics-Uspekhi 59 (11): 1154–1160. doi:10.3367/UFNe.2016.09.037921. Bibcode: 2016PhyU...59.1154E.
- ↑ Semenok, Dmitrii V; Kvashnin, Alexander G; Kruglov, Ivan A; Oganov, Artem R (2018). "Actinium hydrides AcH10, AcH12, AcH16 as high-temperature conventional superconductors". The Journal of Physical Chemistry Letters 9 (8): 1920–1926. doi:10.1021/acs.jpclett.8b00615. PMID 29589444.
- ↑ Sukmas, Wiwittawin; Tsuppayakorn-aek, Prutthipong; Pinsook, Udomsilp; Bovornratanaraks, Thiti (30 December 2020). "Near-room-temperature superconductivity of Mg/Ca substituted metal hexahydride under pressure". Journal of Alloys and Compounds 849: 156434. doi:10.1016/j.jallcom.2020.156434. https://www.sciencedirect.com/science/article/abs/pii/S0925838820327985.
- ↑ Flores-Livas, José A.; Arita, Ryotaro (26 August 2019). "A Prediction for "Hot" Superconductivity". Physics 12: 96. doi:10.1103/Physics.12.96. Bibcode: 2019PhyOJ..12...96F.
- ↑ Grockowiak, A. D.; Ahart, M.; Helm, T.; Coniglio, W. A.; Kumar, R.; Somayazulu, M.; Meng, Y.; Oliff, M. et al. (2022). "Hot Hydride Superconductivity Above 550 K" (in en). Frontiers in Electronic Materials 2. doi:10.3389/femat.2022.837651.
- ↑ Di Cataldo, Simone; von der Linden, Wolfgang; Boeri, Lilia (2021-06-14). "La-$X$-H hydrides: is hot superconductivity possible?". arXiv:2106.07266 [cond-mat.supr-con].
- ↑ Semenok, Dmitrii V.; Troyan, Ivan A.; Ivanova, Anna G.; Kvashnin, Alexander G.; Kruglov, Ivan A.; Hanfland, Michael; Sadakov, Andrey V.; Sobolevskiy, Oleg A. et al. (July 2021). "Superconductivity at 253 K in lanthanum–yttrium ternary hydrides". Materials Today: S1369702121001309. doi:10.1016/j.mattod.2021.03.025.
 |

