Chemistry:Lanthanum decahydride
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| |
| |
| Properties | |
| H10La | |
| Molar mass | 148.985 g·mol−1 |
| Structure[2] | |
| Cubic | |
| Fm3m | |
a = 5.1019(5) Å at 150 GPa
| |
Lattice volume (V)
|
132.80(4) Å3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lanthanum decahydride is a polyhydride or superhydride compound of lanthanum and hydrogen (LaH10) that has shown evidence of being a high-temperature superconductor. It was the first metal superhydride to be theoretically predicted[3][4], synthesized [5], and experimentally confirmed[6] to superconduct at near room-temperatures. It has a superconducting transition temperature TC around 250 K (−23 °C; −10 °F) at a pressure of 150 gigapascals (22×106 psi), and its synthesis required pressures above approximately 160 gigapascals (23×106 psi).[7][8]
Synopsis
Since its discovery, the superconducting properties of LaH10 and other lanthanum-based superhydrides have been experimentally confirmed in multiple independent experiments[9][10][11][12]. The compound exhibits a Meissner effect below the superconducting transition temperature.[13] A cubic form can be synthesized at 1,000 K (730 °C; 1,340 °F),[7] and a hexagonal crystal structure can be formed at room temperature.[14] Further reports indicate Tc is increased with nitrogen doping [15], and decreased with the introduction of magnetic impurities [16].
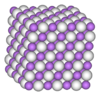
The cubic form has each lanthanum atom surrounded by 32 hydrogen atoms, which form the vertices of an 18-faced shape called a chamfered cube.[17]
A similar compound, lanthanum boron octahydride, was computationally predicted to be a superconductor at 126 K (−147 °C; −233 °F) and pressure 50 gigapascals (7.3×106 psi).[18]
References
- ↑ "Lanthanum decahydride". https://www.acs.org/content/acs/en/molecule-of-the-week/archive/l/lanthanum-decahydride.html.
- ↑ Geballe, Zachary M.; Liu, Hanyu; Mishra, Ajay K.; Ahart, Muhtar; Somayazulu, Maddury; Meng, Yue; Baldini, Maria; Hemley, Russell J. (15 January 2018). "Synthesis and Stability of Lanthanum Superhydrides" (in en). Angewandte Chemie 130 (3): 696–700. doi:10.1002/ange.201709970. ISSN 0044-8249. Bibcode: 2018AngCh.130..696G. https://onlinelibrary.wiley.com/doi/10.1002/ange.201709970.
- ↑ Peng, Feng; Sun, Ying; Pickard, Chris J.; Needs, Richard J.; Wu, Qiang; Ma, Yanming (2017-09-08). "Hydrogen Clathrate Structures in Rare Earth Hydrides at High Pressures: Possible Route to Room-Temperature Superconductivity". Physical Review Letters 119 (10): 107001. doi:10.1103/PhysRevLett.119.107001. PMID 28949166. Bibcode: 2017PhRvL.119j7001P. https://link.aps.org/doi/10.1103/PhysRevLett.119.107001.
- ↑ Liu, Hanyu; Naumov, Ivan I.; Hoffmann, Roald; Ashcroft, N. W.; Hemley, Russell J. (2017-07-03). "Potential high- T c superconducting lanthanum and yttrium hydrides at high pressure" (in en). Proceedings of the National Academy of Sciences 114 (27): 6990–6995. doi:10.1073/pnas.1704505114. ISSN 0027-8424. PMID 28630301.
- ↑ Geballe, Zachary M.; Liu, Hanyu; Mishra, Ajay K.; Ahart, Muhtar; Somayazulu, Maddury; Meng, Yue; Baldini, Maria; Hemley, Russell J. (2018-01-15). "Synthesis and Stability of Lanthanum Superhydrides" (in en). Angewandte Chemie 130 (3): 696–700. doi:10.1002/ange.201709970. ISSN 0044-8249. Bibcode: 2018AngCh.130..696G. https://onlinelibrary.wiley.com/doi/10.1002/ange.201709970.
- ↑ Somayazulu, Maddury; Ahart, Muhtar; Mishra, Ajay K.; Geballe, Zachary M.; Baldini, Maria; Meng, Yue; Struzhkin, Viktor V.; Hemley, Russell J. (2019-01-14). "Evidence for Superconductivity above 260 K in Lanthanum Superhydride at Megabar Pressures". Physical Review Letters 122 (2): 027001. doi:10.1103/PhysRevLett.122.027001. PMID 30720326. Bibcode: 2019PhRvL.122b7001S. https://link.aps.org/doi/10.1103/PhysRevLett.122.027001.
- ↑ 7.0 7.1 Drozdov, A. P.; Kong, P. P.; Minkov, V. S.; Besedin, S. P.; Kuzovnikov, M. A.; Mozaffari, S.; Balicas, L.; Balakirev, F. F. et al. (2019). "Superconductivity at 250 K in lanthanum hydride under high pressures". Nature 569 (7757): 528–531. doi:10.1038/s41586-019-1201-8. PMID 31118520. Bibcode: 2019Natur.569..528D.
- ↑ M. Kostrzewa; K. M. Szczęśniak; A. P. Durajski; R. Szczęśniak (31 January 2020). "From LaH10 to room–temperature superconductors". Scientific Reports 10 (1): 1592. doi:10.1038/s41598-020-58065-9. PMID 32005852. Bibcode: 2020NatSR..10.1592K.
- ↑ Drozdov, A. P.; Kong, P. P.; Minkov, V. S.; Besedin, S. P.; Kuzovnikov, M. A.; Mozaffari, S.; Balicas, L.; Balakirev, F. F. et al. (May 2019). "Superconductivity at 250 K in lanthanum hydride under high pressures" (in en). Nature 569 (7757): 528–531. doi:10.1038/s41586-019-1201-8. ISSN 0028-0836. PMID 31118520. Bibcode: 2019Natur.569..528D. https://www.nature.com/articles/s41586-019-1201-8.
- ↑ Hong, Fang; Yang, Liuxiang; Shan, Pengfei; Yang, Pengtao; Liu, Ziyi; Sun, Jianping; Yin, Yunyu; Yu, Xiaohui et al. (2020-10-01). "Superconductivity of Lanthanum Superhydride Investigated Using the Standard Four-Probe Configuration under High Pressures*". Chinese Physics Letters 37 (10): 107401. doi:10.1088/0256-307X/37/10/107401. ISSN 0256-307X. Bibcode: 2020ChPhL..37j7401H. https://iopscience.iop.org/article/10.1088/0256-307X/37/10/107401.
- ↑ Semenok, Dmitrii V.; Troyan, Ivan A.; Ivanova, Anna G.; Kvashnin, Alexander G.; Kruglov, Ivan A.; Hanfland, Michael; Sadakov, Andrey V.; Sobolevskiy, Oleg A. et al. (2021-09-01). "Superconductivity at 253 K in lanthanum–yttrium ternary hydrides". Materials Today 48: 18–28. doi:10.1016/j.mattod.2021.03.025. ISSN 1369-7021. https://www.sciencedirect.com/science/article/pii/S1369702121001309.
- ↑ Semenok, Dmitrii V.; Troyan, Ivan A.; Sadakov, Andrey V.; Zhou, Di; Galasso, Michele; Kvashnin, Alexander G.; Ivanova, Anna G.; Kruglov, Ivan A. et al. (October 2022). "Effect of Magnetic Impurities on Superconductivity in LaH 10" (in en). Advanced Materials 34 (42): e2204038. doi:10.1002/adma.202204038. ISSN 0935-9648. PMID 35829689. Bibcode: 2022AdM....3404038S. https://onlinelibrary.wiley.com/doi/10.1002/adma.202204038.
- ↑ Eremets, M. I.; Minkov, V. S.; Drozdov, A. P.; Kong, P. P.; Ksenofontov, V.; Shylin, S. I.; Bud'ko, S. L.; Prozorov, R. et al. (10 January 2022). "High‑Temperature Superconductivity in Hydrides: Experimental Evidence and Details". Journal of Superconductivity and Novel Magnetism 35 (4): 965–977. doi:10.1007/s10948-022-06148-1.
- ↑ Geballe, Zachary M.; Liu, Hanyu; Mishra, Ajay K.; Ahart, Muhtar; Somayazulu, Maddury; Meng, Yue; Baldini, Maria; Hemley, Russell J. (15 January 2018). "Synthesis and Stability of Lanthanum Superhydrides". Angewandte Chemie International Edition 57 (3): 688–692. doi:10.1002/anie.201709970. PMID 29193506.
- ↑ Ge, Yanfeng; Zhang, Fan; Hemley, Russell J. (2021-12-15). "Room-temperature superconductivity in boron- and nitrogen-doped lanthanum superhydride". Physical Review B 104 (21): 214505. doi:10.1103/PhysRevB.104.214505. Bibcode: 2021PhRvB.104u4505G. https://link.aps.org/doi/10.1103/PhysRevB.104.214505.
- ↑ Sanner, T. (1975-11-18). "Formation of transient complexes in the glutamate dehydrogenase catalyzed reaction". Biochemistry 14 (23): 5094–5098. doi:10.1021/bi00694a011. ISSN 0006-2960. PMID 39. https://pubmed.ncbi.nlm.nih.gov/39.
- ↑ "NNNS chemistry blog: Lanthanum decahydride". http://chem.vander-lingen.nl/articles/Lanthanum_decahydride/id/894/itemid/895.
- ↑ Di Cataldo, Simone; Heil, Christoph; von der Linden, Wolfgang; Boeri, Lilia (2021-07-29). "BH8: Towards high-Tc low-pressure superconductivity in ternary superhydrides". Physical Review B 104 (2): L020511. doi:10.1103/PhysRevB.104.L020511. Bibcode: 2021PhRvB.104b0511D.
 |