Chemistry:Purpurogallin
From HandWiki
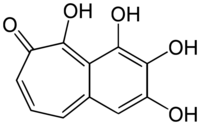
| |
| Names | |
|---|---|
| Preferred IUPAC name
1,7,8,9-Tetrahydroxy-2H-benzo[7]annulen-2-one | |
| Other names
Purpurogalline
2,3,4,6-Tetrahydroxybenzocyclohepten-5-one PPG | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
| MeSH | C026133 |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C11H8O5 | |
| Molar mass | 220.180 g·mol−1 |
| Appearance | Red crystalline solid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Purpurogallin is an aglycone natural product. It is an orange-red solid that is soluble in polar organic solvents but not in water. Its glycoside (ether-linked to sugar), called dryophantin, is found in nutgalls and oak barks. Purpurogallin can be prepared by oxidation of pyrogallol with sodium periodate.[1]
Medicinal aspects
Purpurogallin is bioactive[2] It can inhibit 2-hydroxy and 4-hydroxyestradiol methylation by catechol-O-methyltransferase.[3] It potently and specifically inhibits TLR1/TLR2 activation pathway.[4]
References
- ↑ Kelly-Hunt, Alexandra E.; Mehan, Aman; Brooks, Sarah; Leanca, Miron A.; McKay, Jack E. D.; Mahamed, Nashad; Lambert, Daniel; Dempster, Nicola M. et al. (2022). "Synthesis and Analytical Characterization of Purpurogallin: A Pharmacologically Active Constituent of Oak Galls". Journal of Chemical Education 99 (2): 983–993. doi:10.1021/acs.jchemed.1c00699. Bibcode: 2022JChEd..99..983K. https://researchonline.ljmu.ac.uk/id/eprint/16051/3/Synthesis%20and%20analytical%20characterization%20of%20purpurogallin%20-%20a%20pharmacologically%20active%20constituent%20of%20oak%20galls.pdf.
- ↑ Wu, Tai-Wing; Zeng, Ling-Hua; Wu, Jun; Fung, Kwok-Pui; Weisel, Richard D; Hempel, Andrew; Camerman, Norman (1996). "Molecular structure and antioxidant specificity of purpurogallin in three types of human cardiovascular cells". Biochemical Pharmacology 52 (7): 1073–80. doi:10.1016/0006-2952(96)00447-9. PMID 8831727.
- ↑ Lambert, Joshua D; Chen, Dapeng; Wang, Ching Y; Ai, Ni; Sang, Shengmin; Ho, Chi-Tang; Welsh, William J; Yang, Chung S (2005). "Benzotropolone inhibitors of estradiol methylation: Kinetics and in silico modeling studies". Bioorganic & Medicinal Chemistry 13 (7): 2501–7. doi:10.1016/j.bmc.2005.01.037. PMID 15755652.
- ↑ Cheng, Kui; Wang, Xiaohui; Zhang, Shuting; Yin, Hang (2012). "Discovery of Small-Molecule Inhibitors of the TLR1/TLR2 Complex". Angewandte Chemie International Edition 51 (49): 12246–9. doi:10.1002/anie.201204910. PMID 22969053.
 |

