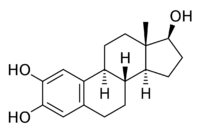
Chemistry:2-Hydroxyestradiol
| |
| Names | |
|---|---|
| IUPAC name
Estra-1,3,5(10)-triene-2,3,17β-triol
| |
| Systematic IUPAC name
(1S,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,7,8-triol | |
| Other names
2-OHE2
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C18H24O3 | |
| Molar mass | 288.387 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2-Hydroxyestradiol (2-OHE2), also known as estra-1,3,5(10)-triene-2,3,17β-triol, is an endogenous steroid, catechol estrogen, and metabolite of estradiol, as well as a positional isomer of estriol.[1]
Biosynthesis
Transformation of estradiol to 2-hydroxyestradiol is a major metabolic pathway of estradiol in the liver.[1] CYP1A2 and CYP3A4 are the major enzymes catalyzing the 2-hydroxylation of estradiol.[1] Conversion of estradiol into 2-hydroxyestradiol has also been detected in the uterus, breast, kidney, brain, and pituitary gland, as well as the placenta, and may similarly be mediated by cytochrome P450 enzymes.[1] Although estradiol is extensively converted into 2-hydroxyestradiol, circulating levels of 2-hydroxyestradiol and levels of 2-hydroxyestradiol in various tissues are very low.[1] This may be due to rapid conjugation (O-methylation, glucuronidation, sulfonation) of 2-hydroxyestradiol followed by urinary excretion.[1]
Biological activity
Estrogenic activity
2-Hydroxyestradiol has approximately 7% and 11% of the affinity of estradiol at the estrogen receptors (ERs) ERα and ERβ, respectively.[2] It dissociates from the estrogen receptors more rapidly than does estradiol.[3] The steroid is only very weakly estrogenic, and is able to antagonize the estrogenic effects of estradiol, indicating that its intrinsic activity at the estrogen receptor is less than that of estradiol and hence that it possesses the profile of a selective estrogen receptor modulator.[1] It shows estrogenic activity in human breast cancer cells.[4] In addition to its activity at the nuclear ERs, 2-hydroxyestradiol is an antagonist of the G protein-coupled estrogen receptor (GPER) (100–1,000 μM).[5]
Catecholaminergic activity
2-Hydroxyestradiol is a catechol estrogen and in this regard bears some structural resemblance to the catecholamines dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline).[6] In accordance, 2-hydroxyestradiol has been found to interact with catecholamine systems.[6] The steroid is known to compete with catecholamines for binding to catechol O-methyltransferase and tyrosine hydroxylase and to directly and competitively inhibit these enzymes.[6][7] In addition, 2-hydroxyestradiol has been found to displace spiperone from the D2 receptor with approximately 50% of the affinity of dopamine, whereas estradiol, estrone, and estriol and their other 2-hydroxylated and 2-methoxylated derivatives showed only weak or negligible inhibition.[6] Moreover, 2-hydroxyestradiol has been found to bind to the α1-adrenergic receptor with slightly more than half the affinity of norepinephrine.[8] However, although these affinities are comparable to those of dopamine and norepinephrine, they are nonetheless in the double-digit micromolar range.[6][8]
2-Hydroxyestradiol has been found to increase prolactin secretion when administered intravenously to women.[9] It was noted that this could be due to 2-hydroxyestradiol binding to and antagonizing the D2 receptor.[9] However, the researchers argued against this possibility because it was delayed (by several hours) and of relatively small magnitude, whereas established D2 receptor antagonists promptly induce marked increases in prolactin levels.[9] The researchers also argued against the possibility that it was due to inhibition of dopamine biosynthesis by 2-hydroxyestradiol because 2-hydroxyestrone, which inhibits tyrosine hydroxylase similarly to 2-hydroxyestradiol, showed no such increase in prolactin secretion.[9] The researchers concluded that the most likely explanation was that the increase was mediated by the estrogenic activity of 2-hydroxyestradiol, as similar increments in prolactin levels had been observed with estradiol.[9] In any case, these findings argue against the notion of major interactions of 2-hydroxyestradiol with the dopamine system.[9]
Genotoxicity
2-Hydroxyestradiol, as well as 2-hydroxyestrone and 4-hydroxyestradiol, can undergo metabolic redox cycling to generate free radicals like superoxide and reactive estrogen semiquinone/quinone intermediates.[1] These metabolites may damage DNA and other cellular components.[1] However, 2-hydroxyestradiol shows little or no tumorigenic activity in the male Syrian hamster kidney and there is evidence that 2-hydroxyestradiol may actually decrease tumorigenesis in estrogen-sensitive tissues.[1] It has been suggested that the lack of tumorigenesis of 2-hydroxyestrone is due to its rapid clearance.[1] In addition, its metabolite 2-methoxyestradiol is a very potent inhibitor of tumor growth and angiogenesis, and this may contribute as well.[1]
Production of 2-methoxyestradiol
2-Hydroxyestradiol has been identified as a prodrug of 2-methoxyestradiol, a transformation which is very efficiently catalyzed by catechol O-methyltransferase in the liver.[10] 2-Methoxyestradiol is not estrogenic but is a potent angiogenesis inhibitor and agonist of the GPER with potential therapeutic implications in cancer.[11]
Antioxidant activity
Similarly to other steroidal estrogens, 2-hydroxyestradiol is an antioxidant, but the catechol estrogens (2- and 4-hydroxylated estrogens) like 2-hydroxyestradiol are considered to be the most potent in terms of antioxidant activity.[12][dubious ]
History
2-Hydroxyestradiol was identified as a metabolite of estradiol in 1960.[13]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 "Functional role of estrogen metabolism in target cells: review and perspectives". Carcinogenesis 19 (1): 1–27. 1998. doi:10.1093/carcin/19.1.1. PMID 9472688.
- ↑ "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology 138 (3): 863–70. 1997. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ↑ "Kinetics of catechol estrogen-estrogen receptor dissociation: a possible factor underlying differences in catechol estrogen biological activity". Steroids 41 (5): 643–56. May 1983. doi:10.1016/0039-128x(83)90030-2. PMID 6658896.
- ↑ "Catecholestrogens are MCF-7 cell estrogen receptor agonists". J. Steroid Biochem. Mol. Biol. 46 (6): 781–9. December 1993. doi:10.1016/0960-0760(93)90319-r. PMID 8274412.
- ↑ "International Union of Basic and Clinical Pharmacology. XCVII. G Protein-Coupled Estrogen Receptor and Its Pharmacologic Modulators". Pharmacol. Rev. 67 (3): 505–40. July 2015. doi:10.1124/pr.114.009712. PMID 26023144.
- ↑ 6.0 6.1 6.2 6.3 6.4 "2-Hydroxyestradiol interaction with dopamine receptor binding in rat anterior pituitary". J. Biol. Chem. 254 (13): 5606–8. 1979. doi:10.1016/S0021-9258(18)50455-5. PMID 447670.
- ↑ "The possible role of 2-hydroxyestradiol in the development of estrogen-induced striatal dopamine receptor hypersensitivity". Brain Res. 333 (1): 1–10. 1985. doi:10.1016/0006-8993(85)90117-9. PMID 2986765.
- ↑ 8.0 8.1 "Competition by estrogens for catecholamine receptor binding in vitro". J. Neurochem. 39 (2): 512–20. 1982. doi:10.1111/j.1471-4159.1982.tb03974.x. PMID 7086432.
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 "Stimulatory effect of 2-hydroxyestradiol on prolactin release in hypogonadal women". J. Clin. Endocrinol. Metab. 51 (2): 413–5. 1980. doi:10.1210/jcem-51-2-413. PMID 6772666.
- ↑ "The tsunami of tuberculosis". Med. J. Aust. 182 (6): 263–4. 2005. doi:10.5694/j.1326-5377.2005.tb06696.x. PMID 15777138.
- ↑ Thekkumkara, Thomas; Snyder, Russell; Karamyan, Vardan T. (2016). "Competitive Binding Assay for the G-Protein-Coupled Receptor 30 (GPR30) or G-Protein-Coupled Estrogen Receptor (GPER)". Estrogen Receptors. Methods in Molecular Biology. 1366. pp. 11–17. doi:10.1007/978-1-4939-3127-9_2. ISBN 978-1-4939-3126-2.
- ↑ Gabor M. Rubanyi; R Kauffman (2 September 2003). Estrogen and the Vessel Wall. CRC Press. pp. 88–. ISBN 978-0-203-30393-1. https://books.google.com/books?id=U6WU25VeOBsC&pg=PA88.
- ↑ "Metabolism of estrogens--natural and synthetic". Pharmacol. Ther. 4 (1): 155–81. 1979. doi:10.1016/0163-7258(79)90018-4. PMID 379882.
 |
