Chemistry:Sodium periodate
| |
| Names | |
|---|---|
| IUPAC name
Sodium periodate
| |
| Other names
Sodium metaperiodate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| |
| |
| Properties | |
| NaIO4 | |
| Molar mass | 213.8918 g/mol |
| Appearance | white crystals |
| Density | 3.865 g/cm3 (anhydrous) 3/210 g/cm3 |
| Melting point | 300 °C (572 °F; 573 K) (anhydrous) 175 °C (347 °F; 448 K) (trihydrate) (decomposes) |
| 91 g/L[1] | |
| Solubility | soluble in acids |
| Structure | |
| tetragonal (anhydrous) trigonal (trihydrate) | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Related compounds | |
Other anions
|
sodium perchlorate, sodium perbromate |
Other cations
|
potassium periodate, periodic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium periodate is an inorganic salt, composed of a sodium cation and the periodate anion. It may also be regarded as the sodium salt of periodic acid. Like many periodates, it can exist in two different forms: sodium metaperiodate (formula NaIO4) and sodium orthoperiodate (normally Na2H3IO6, but sometimes the fully reacted salt Na5IO6). Both salts are useful oxidising agents.[2]
Preparation
Classically, periodate was most commonly produced in the form of sodium hydrogen periodate (Na
3H
2IO
6).[3] This is commercially available, but can also be produced by the oxidation of iodates with chlorine and sodium hydroxide.[4] Or, similarly, from iodides by oxidation with bromine and sodium hydroxide:
- [math]\ce{ \overset{sodium\ iodate}{NaIO3} + Cl2{} + 4 NaOH -> Na3H2IO6{} + 2NaCl{} + H2O }[/math]
- [math]\ce{ NaI + 4 Br2 + 10 NaOH -> Na3H2IO6 + 8 NaBr + 4 H2O }[/math]
Modern industrial scale production involves the electrochemical oxidation of iodates, on a lead dioxide (PbO
2) anode, with the following standard electrode potential:
Sodium metaperiodate can be prepared by the dehydration of sodium hydrogen periodate with nitric acid.[3]
- [math]\ce{ Na3H2IO6 + 2 HNO3 -> NaIO4 + 2 NaNO3 + 2 H2O }[/math]
Structure
Sodium metaperiodate (NaIO4) forms tetragonal crystals (space group I41/a) consisting of slightly distorted IO−4 ions with average I–O bond distances of 1.775 Å; the Na+ ions are surrounded by 8 oxygen atoms at distances of 2.54 and 2.60 Å.[6]
Sodium hydrogen periodate (Na2H3IO6) forms orthorhombic crystals (space group Pnnm). Iodine and sodium atoms are both surrounded by an octahedral arrangement of 6 oxygen atoms; however the NaO6 octahedron is strongly distorted. IO6 and NaO6 groups are linked via common vertices and edges.[7]
Powder diffraction indicates that Na5IO6 crystallises in the monoclinic system (space group C2/m).[8]
Uses
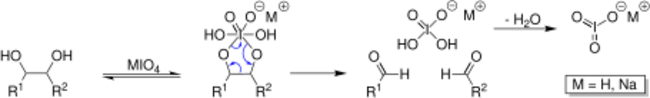
Sodium periodate can be used in solution to open saccharide rings between vicinal diols leaving two aldehyde groups. This process is often used in labeling saccharides with fluorescent molecules or other tags such as biotin. Because the process requires vicinal diols, periodate oxidation is often used to selectively label the 3′-ends of RNA (ribose has vicinal diols) instead of DNA as deoxyribose does not have vicinal diols.
NaIO4 is used in organic chemistry to cleave diols to produce two aldehydes.[9]
In 2013 the US Army announced that it would replace environmentally harmful chemicals barium nitrate and potassium perchlorate with sodium metaperiodate for use in their tracer ammunition.[10]
See also
- lead tetraacetate - also effective for diol cleavage via the Criegee oxidation
References
- ↑ Record of Natriumperiodat in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2018-01-08.
- ↑ Andrew G. Wee, Jason Slobodian, Manuel A. Fernández-Rodríguez and Enrique Aguilar "Sodium Periodate" e-EROS Encyclopedia of Reagents for Organic Synthesis 2006. doi:10.1002/047084289X.rs095.pub2
- ↑ 3.0 3.1 Riley, edited by Georg Brauer ; translated by Scripta Technica, Inc. Translation editor Reed F. (1963). Handbook of preparative inorganic chemistry. Volume 1 (2nd ed.). New York, N.Y.: Academic Press. pp. 323–324. ISBN 012126601X.
- ↑ Hill, Arthur E. (October 1928). "Ternary Systems. VII. The Periodates of the Alkali Metals". Journal of the American Chemical Society 50 (10): 2678–2692. doi:10.1021/ja01397a013.
- ↑ Parsons, Roger (1959). Handbook of electrochemical constants. Butterworths Scientific Publications Ltd. p. 71. https://archive.org/details/ost-chemistry-parsons-handbookofelectrochemicalconstants.
- ↑ Kálmán, A.; Cruickshank, D. W. J. (15 November 1970). "Refinement of the structure of NaIO4". Acta Crystallographica Section B 26 (11): 1782–1785. doi:10.1107/S0567740870004880. Bibcode: 1970AcCrB..26.1782K.
- ↑ Jansen, Martin; Rehr, Anette (1988). "Na2H3IO6, eine Variante der Markasitstruktur" (in de). Zeitschrift für anorganische und allgemeine Chemie 567 (1): 95–100. doi:10.1002/zaac.19885670111.
- ↑ Betz, T.; Hoppe, R. (May 1984). "Über Perrhenate. 2. Zur Kenntnis von Li5ReO6 und Na5ReO6 – mit einer Bemerkung über Na5IO6" (in de). Zeitschrift für anorganische und allgemeine Chemie 512 (5): 19–33. doi:10.1002/zaac.19845120504.
- ↑ McMurry, John. Organic chemistry (8th ed., [international ed.] ed.). Singapore: Brooks/Cole Cengage Learning. pp. 285–286. ISBN 9780840054531.
- ↑ "Picatinny to remove tons of toxins from lethal rounds". U.S. Army. http://www.army.mil/article/109769/Picatinny_to_remove_tons_of_toxins_from_lethal_rounds/.
- See Fatiadi, Synthesis (1974) 229–272 for a review of periodate chemistry.
 |