Medicine:Marrow adipose tissue
| Marrow adipose tissue | |
|---|---|
 | |
| Anatomical terminology |
Marrow adipose tissue (MAT), also known as bone marrow adipose tissue (BMAT), is a type of fat deposit in bone marrow. It increases in states of low bone density -osteoporosis,[1][2] anorexia nervosa/ caloric restriction,[3][4] skeletal unweighting such as that which occurs in space travel,[5][6] and anti-diabetes therapies.[7]
Origin
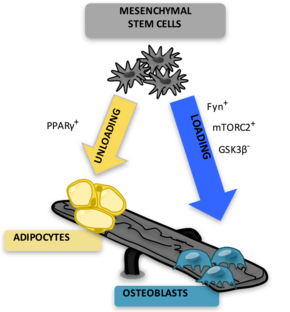
The marrow adipocytes originate from mesenchymal stem cell (MSC) progenitors that also give rise to osteoblasts, among other cell types.[8] Thus, it is thought that MAT results from preferential MSC differentiation into the adipocyte, rather than osteoblast, lineage in the setting of osteoporosis.[9] Since MAT is increased in the setting of obesity[10][11][12] and is suppressed by endurance exercise,[13][10][14][15] or vibration,[16] it is likely that MAT physiology, in the setting of mechanical input/exercise, approximates that of white adipose tissue (WAT).
Exercise regulation of marrow adipose tissue
The first study to demonstrate exercise regulation of MAT in rodents was published in 2014;[10] Now, exercise regulation of MAT has been confirmed in a humansl[17] adding clinical importance. Several studies demonstrated exercise reduction of MAT which occurs along with an increase in bone quantity.[15][13][14][18] Since exercise increases bone quantity, reduces MAT and increases expression of markers of fatty acid oxidation in bone, MAT is thought to be providing needed fuel for exercise-induced bone formation or anabolism.[14] One notable exception occurs in the setting of caloric restriction: exercise suppression of MAT does not yield an increase in bone formation and even appears to cause bone loss.[4][19][18] Indeed, energy availability appears to be a factor in the ability of exercise to regulate MAT.[citation needed]
Relationships to other types of fat
MAT has qualities of both white and brown fat.[20] Subcutaneous white fat contain excess energy, indicating a clear evolutionary advantage during times of scarcity. WAT is also the source of adipokines and inflammatory markers which have both positive (e.g., adiponectin)[21] and negative[22] effects on metabolic and cardiovascular endpoints. Visceral abdominal fat (VAT) is a distinct type of WAT that is "proportionally associated with negative metabolic and cardiovascular morbidity",[23] regenerates cortisol,[24] and recently has been tied to decreased bone formation[25][26] Both types of WAT substantially differ from brown adipose tissue (BAT) as by a group of proteins that help BAT's thermogenic role.[27] MAT, by its "specific marrow location, and its adipocyte origin from at least LepR+ marrow MSC is separated from non-bone fat storage by larger expression of bone transcription factors",[28] and likely indicates a different fat phenotype.[29] Recently, MAT was noted to "produce a greater proportion of adiponectin – an adipokine associated with improved metabolism – than WAT",[30] suggesting an endocrine function for this depot, akin, but different, from that of WAT.
Impact on bone health
MAT increases in states of bone fragility. MAT is thought to result from preferential MSC differentiation into an adipocyte, rather than osteoblast lineage in osteoporosis[9][18] based on the inverse relationship between bone and MAT in bone-fragile osteoporotic states. An increase in MAT is noted in osteoporosis clinical studies measured by MR Spectroscopy.[31][32][33] Estrogen therapy in postmenopausal osteoporosis reduces MAT.[34] Antiresorptive therapies like risedronate or zoledronate also decrease MAT while increasing bone density, supporting an inverse relationship between bone quantity and MAT. During aging, bone quantity declines[35][36] and fat redistributes from subcutaneous to ectopic sites such as bone marrow, muscle, and liver.[37] Aging is associated with lower osteogenic and greater adipogenic biasing of MSC.[38] This aging-related biasing of MSC away from osteoblast lineage may represent higher basal PPARγ expression[39] or decreased Wnt10b.[40][41][42] Thus, bone fragility, osteoporosis, and osteoporotic fractures are thought to be linked to mechanisms which promote MAT accumulation.[citation needed]
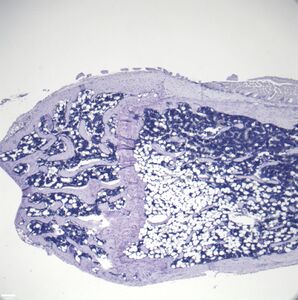
- Histologic sections demonstrating Marrow Adipocytes
Maintenance of hematopoietic stem cells
Bone marrow adipocytes secrete factors that promote HSC renewal in most bones.[43]
Hematopoietic cells (also known as blood cells) reside in the bone marrow along with marrow adipocytes. These hematopoietic cells are derived from hematopoietic stem cells (HSC) which give rise to diverse cells: cells of the blood, immune system, as well as cells that break down bone (osteoclasts). HSC renewal occurs in the marrow stem cell niche, a microenvironment that contains cells and secreted factors that promote appropriate renewal and differentiation of HSC. The study of the stem cell niche is relevant to the field of oncology in order to improve therapy for multiple hematologic cancers. As such cancers are often treated with bone marrow transplantation, there is interest in improving the renewal of HSC.[citation needed]
Measurement
In order to understand the physiology of MAT, various analytic methods have been applied. Marrow adipocytes are difficult to isolate and quantify because they are interspersed with bony and hematopoietic elements. Until recently, qualitative measurements of MAT have relied on bone histology,[44][45] which is subject to site selection bias and cannot adequately quantify the volume of fat in the marrow. Nevertheless, histological techniques and fixation make possible visualization of MAT, quantification of adipocyte size, and MAT's association with the surrounding endosteum, milieu of cells, and secreted factors.[46][47][48]
Recent advances in cell surface and intracellular marker identification and single-cell analyses led to greater resolution and high-throughput ex-vivo quantification. Flow cytometric quantification can be used to purify adipocytes from the stromal vascular fraction of most fat depots.[49] Early research with such machinery cited adipocytes as too large and fragile for cytometer-based purification, rendering them susceptible to lysis; however, recent advances have been made to mitigate this;[50] nevertheless, this methodology continues to pose technical challenges[51] and is inaccessible to much of the research community.
To improve quantification of MAT, novel imaging techniques have been developed as a means to visualize and quantify MAT. Although proton magnetic resonance spectroscopy (1H-MRS) has been used with success to quantify vertebral MAT in humans,[52] it is difficult to employ in laboratory animals.[53] Magnetic resonance imaging (MRI) provides MAT assessment in the vertebral skeleton[54] in conjunction with μCT-based marrow density measurements.[55] A volumetric method to identify, quantify, and localize MAT in rodent bone has been recently developed, requiring osmium staining of bones and μCT imaging,[56] followed by advanced image analysis of osmium-bound lipid volume (in mm3) relative to bone volume.[10][14][13] This technique provides reproducible quantification and visualization of MAT, enabling the ability to consistently quantify changes in MAT with diet, exercise, and agents that constrain precursor lineage allocation. Although the osmium method is quantitatively precise, osmium is toxic and cannot be compared across batched experiments. Recently, researchers developed and validated[14] a 9.4T MRI scanner technique that allows localization and volumetric (3D) quantification that can be compared across experiments, as in.[4]
- Methods for Quantification of Marrow Adipose Tissue (MAT)
Figure. This figure demonstrates the use of the osmium- μCT method with advanced image processing to quantify MAT. In this figure, running exercise is shown to suppress MAT despite PPARγ agonist. Fat binder osmium is imaged via μCT (A ) in n =5 per group overlaid images. Quantification of osmium as MAT/ bone volume in the whole femur is shown. a, significant due to Rosi. b, significant due to exercise. Rosi=rosiglizaone, CTL=control, E=exercise.
Figure. This demonstrates the use of MRI imaging (9.4T scanner) along with advanced image processing to quantify MAT. The images and graph demonstrate that MAT is higher in obese compared with lean mice. B6 mice were fed HFD from age 4 wk until age 16 wk. MAT was quantified by MRI. A) n=10 superimposed group average images are shown B) MAT normalized to bone volume in each group.
References
- ↑ "Increased marrow adiposity in premenopausal women with idiopathic osteoporosis". The Journal of Clinical Endocrinology and Metabolism 97 (8): 2782–91. August 2012. doi:10.1210/jc.2012-1477. PMID 22701013.
- ↑ "Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies". Clinical Orthopaedics and Related Research 80: 147–54. October 1971. doi:10.1097/00003086-197110000-00021. PMID 5133320.
- ↑ "Marrow fat and bone--new perspectives". The Journal of Clinical Endocrinology and Metabolism 98 (3): 935–45. March 2013. doi:10.1210/jc.2012-3634. PMID 23393168.
- ↑ 4.0 4.1 4.2 "Exercise Degrades Bone in Caloric Restriction, Despite Suppression of Marrow Adipose Tissue (MAT)". Journal of Bone and Mineral Research 35 (1): 106–115. January 2020. doi:10.1002/jbmr.3872. PMID 31509274.
- ↑ "Transforming growth factor beta2 inhibits adipocyte differentiation induced by skeletal unloading in rat bone marrow stroma". Journal of Bone and Mineral Research 17 (4): 668–77. April 2002. doi:10.1359/jbmr.2002.17.4.668. PMID 11918224.
- ↑ "Skeletal abnormalities in rats induced by simulated weightlessness". Metabolic Bone Disease & Related Research 4 (1): 69–75. 1982-01-01. doi:10.1016/0221-8747(82)90011-X. PMID 7121257.
- ↑ "Effects of rosiglitazone vs metformin on circulating osteoclast and osteogenic precursor cells in postmenopausal women with type 2 diabetes mellitus". The Journal of Clinical Endocrinology and Metabolism 99 (10): E1933-42. October 2014. doi:10.1210/jc.2013-3666. PMID 24905061.
- ↑ "Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: cross talk with the osteoblastogenic program". Cellular and Molecular Life Sciences 66 (2): 236–53. January 2009. doi:10.1007/s00018-008-8429-z. PMID 18854943.
- ↑ 9.0 9.1 "The Role of Bone Marrow Fat in Skeletal Health: Usefulness and Perspectives for Clinicians". The Journal of Clinical Endocrinology and Metabolism 100 (10): 3613–21. October 2015. doi:10.1210/jc.2015-2338. PMID 26244490.
- ↑ 10.0 10.1 10.2 10.3 "Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise". Bone 64: 39–46. July 2014. doi:10.1016/j.bone.2014.03.044. PMID 24709686.
- ↑ "Changes in Skeletal Integrity and Marrow Adiposity during High-Fat Diet and after Weight Loss". Frontiers in Endocrinology 7: 102. 2016. doi:10.3389/fendo.2016.00102. PMID 27512386.
- ↑ "A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice". Journal of Cellular Physiology 230 (9): 2032–7. September 2015. doi:10.1002/jcp.24954. PMID 25663195.
- ↑ 13.0 13.1 13.2 "Exercise Regulation of Marrow Fat in the Setting of PPARγ Agonist Treatment in Female C57BL/6 Mice". Endocrinology 156 (8): 2753–61. August 2015. doi:10.1210/en.2015-1213. PMID 26052898.
- ↑ 14.0 14.1 14.2 14.3 14.4 "Exercise Decreases Marrow Adipose Tissue through β-Oxidation in Obese Running Mice". Journal of Bone and Mineral Research 32 (8): 1692–1702. August 2017. doi:10.1002/jbmr.3159. PMID 28436105.
- ↑ 15.0 15.1 "Combating osteoporosis and obesity with exercise: leveraging cell mechanosensitivity". Nature Reviews. Endocrinology 15 (6): 339–355. June 2019. doi:10.1038/s41574-019-0170-1. PMID 30814687.
- ↑ "Mechanical Signals As a Non-Invasive Means to Influence Mesenchymal Stem Cell Fate, Promoting Bone and Suppressing the Fat Phenotype". BoneKEy Osteovision 6 (4): 132–149. April 2009. doi:10.1138/20090371. PMID 22241295.
- ↑ "Specific Modulation of Vertebral Marrow Adipose Tissue by Physical Activity". Journal of Bone and Mineral Research 33 (4): 651–657. April 2018. doi:10.1002/jbmr.3357. PMID 29336053.
- ↑ 18.0 18.1 18.2 Little-Letsinger, Sarah E.; Pagnotti, Gabriel M.; McGrath, Cody; Styner, Maya (2020-10-17). "Exercise and Diet: Uncovering Prospective Mediators of Skeletal Fragility in Bone and Marrow Adipose Tissue" (in en). Current Osteoporosis Reports 18 (6): 774–789. doi:10.1007/s11914-020-00634-y. ISSN 1544-1873. PMID 33068251.
- ↑ Southmayd, Emily A; Williams, Nancy I; Mallinson, Rebecca J; De Souza, Mary Jane (2019-03-21). "Energy Deficiency Suppresses Bone Turnover in Exercising Women With Menstrual Disturbances". The Journal of Clinical Endocrinology & Metabolism 104 (8): 3131–3145. doi:10.1210/jc.2019-00089. ISSN 0021-972X. PMID 30896746.
- ↑ "Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes". Bone 50 (2): 546–52. February 2012. doi:10.1016/j.bone.2011.06.016. PMID 21723971.
- ↑ "Adiponectin, driver or passenger on the road to insulin sensitivity?". Molecular Metabolism 2 (3): 133–41. April 2013. doi:10.1016/j.molmet.2013.04.001. PMID 24049728.
- ↑ "Adipocytokines: mediators linking adipose tissue, inflammation and immunity". Nature Reviews. Immunology 6 (10): 772–83. October 2006. doi:10.1038/nri1937. PMID 16998510.
- ↑ "Structural and biochemical characteristics of various white adipose tissue depots". Acta Physiologica 205 (2): 194–208. June 2012. doi:10.1111/j.1748-1716.2012.02409.x. PMID 22226221.
- ↑ "A transgenic model of visceral obesity and the metabolic syndrome". Science 294 (5549): 2166–70. December 2001. doi:10.1126/science.1066285. PMID 11739957. Bibcode: 2001Sci...294.2166M.
- ↑ "Determinants of bone microarchitecture and mechanical properties in obese men". The Journal of Clinical Endocrinology and Metabolism 97 (11): 4115–22. November 2012. doi:10.1210/jc.2012-2246. PMID 22933540.
- ↑ "Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study". The Journal of Clinical Endocrinology and Metabolism 98 (6): 2562–72. June 2013. doi:10.1210/jc.2013-1047. PMID 23515452.
- ↑ "Adaptive thermogenesis in adipocytes: is beige the new brown?". Genes & Development 27 (3): 234–50. February 2013. doi:10.1101/gad.211649.112. PMID 23388824.
- ↑ "Human stromal (mesenchymal) stem cells from bone marrow, adipose tissue and skin exhibit differences in molecular phenotype and differentiation potential". Stem Cell Reviews and Reports 9 (1): 32–43. February 2013. doi:10.1007/s12015-012-9365-8. PMID 22529014.
- ↑ "Playing with bone and fat". Journal of Cellular Biochemistry 98 (2): 251–66. May 2006. doi:10.1002/jcb.20777. PMID 16479589.
- ↑ "Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction". Cell Metabolism 20 (2): 368–375. August 2014. doi:10.1016/j.cmet.2014.06.003. PMID 24998914.
- ↑ "Effects of risedronate on bone marrow adipocytes in postmenopausal women". Osteoporosis International 22 (5): 1547–53. May 2011. doi:10.1007/s00198-010-1353-8. PMID 20661545.
- ↑ "Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: a proton MR spectroscopy study". Journal of Magnetic Resonance Imaging 22 (2): 279–85. August 2005. doi:10.1002/jmri.20367. PMID 16028245.
- ↑ "Quantification of vertebral bone marrow fat content using 3 Tesla MR spectroscopy: reproducibility, vertebral variation, and applications in osteoporosis". Journal of Magnetic Resonance Imaging 33 (4): 974–9. April 2011. doi:10.1002/jmri.22489. PMID 21448966.
- ↑ "Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women". Osteoporosis International 19 (9): 1323–30. September 2008. doi:10.1007/s00198-008-0574-6. PMID 18274695.
- ↑ "Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment". Journal of Bone and Mineral Research 21 (1): 124–31. January 2006. doi:10.1359/jbmr.050916. PMID 16355281.
- ↑ "Age-related changes in trabecular architecture differ in female and male C57BL/6J mice". Journal of Bone and Mineral Research 22 (8): 1197–207. August 2007. doi:10.1359/jbmr.070507. PMID 17488199.
- ↑ "Fat tissue, aging, and cellular senescence". Aging Cell 9 (5): 667–84. October 2010. doi:10.1111/j.1474-9726.2010.00608.x. PMID 20701600.
- ↑ "Senescence-associated intrinsic mechanisms of osteoblast dysfunctions". Aging Cell 10 (2): 191–7. April 2011. doi:10.1111/j.1474-9726.2011.00669.x. PMID 21210937.
- ↑ "Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways". Aging Cell 3 (6): 379–89. December 2004. doi:10.1111/j.1474-9728.2004.00127.x. PMID 15569355.
- ↑ "Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells". Journal of Bone and Mineral Research 25 (10): 2138–47. October 2010. doi:10.1002/jbmr.118. PMID 20499361.
- ↑ "Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation". Journal of Bone and Mineral Research 22 (12): 1924–32. December 2007. doi:10.1359/jbmr.070810. PMID 17708715.
- ↑ "Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?". Cell Death and Differentiation 23 (7): 1128–39. July 2016. doi:10.1038/cdd.2015.168. PMID 26868907.
- ↑ "Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF". Nature Cell Biology 19 (8): 891–903. August 2017. doi:10.1038/ncb3570. PMID 28714970.
- ↑ "Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats". Journal of Bone and Mineral Research 25 (2): 275–84. February 2010. doi:10.1359/jbmr.090813. PMID 19653818.
- ↑ "Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading". Journal of Bone and Mineral Research 28 (4): 865–74. April 2013. doi:10.1002/jbmr.1807. PMID 23109229.
- ↑ "Congenic mice with low serum IGF-I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program". Bone 35 (5): 1046–58. November 2004. doi:10.1016/j.bone.2004.07.008. PMID 15542029.
- ↑ "Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment". Nature 460 (7252): 259–63. July 2009. doi:10.1038/nature08099. PMID 19516257. Bibcode: 2009Natur.460..259N.
- ↑ "Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis". Endocrinology 148 (5): 2553–62. May 2007. doi:10.1210/en.2006-1704. PMID 17317771.
- ↑ "Adipose lineage specification of bone marrow-derived myeloid cells". Adipocyte 1 (4): 215–229. October 2012. doi:10.4161/adip.21496. PMID 23700536.
- ↑ "Analysis and isolation of adipocytes by flow cytometry". Methods of Adipose Tissue Biology, Part A. Methods in Enzymology. 537. 2014. pp. 281–96. doi:10.1016/b978-0-12-411619-1.00015-x. ISBN 9780124116191.
- ↑ "Flow cytometric analysis of mature adipocytes". Cytometry 10 (4): 469–74. July 1989. doi:10.1002/cyto.990100416. PMID 2766892.
- ↑ "Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women". Obesity 19 (1): 49–53. January 2011. doi:10.1038/oby.2010.106. PMID 20467419.
- ↑ "VDR haploinsufficiency impacts body composition and skeletal acquisition in a gender-specific manner". Calcified Tissue International 89 (3): 179–91. September 2011. doi:10.1007/s00223-011-9505-1. PMID 21637996.
- ↑ "Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa". Journal of Bone and Mineral Research 27 (9): 1864–71. September 2012. doi:10.1002/jbmr.1640. PMID 22508185.
- ↑ "Differential effects of exercise on tibial shaft marrow density in young female athletes". The Journal of Clinical Endocrinology and Metabolism 98 (5): 2037–44. May 2013. doi:10.1210/jc.2012-3748. PMID 23616150.
- ↑ "Use of osmium tetroxide staining with microcomputerized tomography to visualize and quantify bone marrow adipose tissue in vivo". Methods of Adipose Tissue Biology, Part A. Methods in Enzymology. 537. 2014. pp. 123–39. doi:10.1016/b978-0-12-411619-1.00007-0. ISBN 9780124116191.
Further reading
- "Bone marrow fat tissue secretes hormone that helps body stay healthy". University of Michigan. 3 July 2014. http://www.uofmhealth.org/news/archive/201407/bone-marrow-fat-tissue-secretes-hormone-helps-body-stay.
- "Another reason to exercise: Burning bone fat a key to better bone health". Science Daily. 18 May 2017. https://www.sciencedaily.com/releases/2017/05/170518140220.htm.