Chemistry:Rhodamine 123
| |
| Names | |
|---|---|
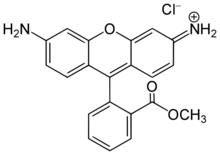
| Preferred IUPAC name
7-Amino-10-[2-(methoxycarbonyl)phenyl]-2H-xanthene-2-iminium chloride | |
| Other names
Rhodamine 123, EINECS 263-687-8, RH 123, LS-162564, C11190
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H17ClN2O3 | |
| Molar mass | 380.824 |
| Melting point | 235 °C (455 °F; 508 K) |
| Solubility in ethanol | 20 g/l |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+312, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhodamine 123 /ˈroʊdəmiːn/ is a chemical compound and a dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with instruments called fluorometers. Rhodamine dyes are used extensively in biotechnology applications such as fluorescence microscopy, flow cytometry, fluorescence correlation spectroscopy and ELISA. Rhodamine fluorescence can also be used as a measure of membrane polarization in live cell assays both within mitochondria[1][2] and with bacteria. This use relies on the fact that rhodamine 123 accumulates in membranes in a manner which is dependent on membrane polarization.[3]
The absorption of rhodamine 123 peaks around 505 nm and luminescence is tunable around 560 nm when used as a laser dye.[4] Its luminescence quantum yield is 0.90.[5]
References
- ↑ L. B. Chen. "Mitochondrial membrane potential in living cells."Annu Rev Cell Biol. 4 (1988) 155–181
- ↑ Darzynkiewicz Z, Traganos F, Staiano-Coico L, Kapuscinski J, Melamed MR. (1982) Interaction of rhodamine 123 with living cells studied by flow cytometry. Cancer Res. Mar;42(3):799-806. PMID 7059978
- ↑ M. Huang, A. K. S. Camara, D. F. Stowe, F. Qi, D. A. Beard. "Mitochondrial inner membrane electrophysiology assessed by rhodamine-123 transport and fluorescence" Annals of Biomedical Engineering. (2007) 35 (7)
- ↑ Rhodamine 123
- ↑ R. F. Kubin and A. N. Fletcher, "Fluorescence quantum yields of some rhodamine dyes." J. Luminescence 27 (1982) 455
See also
 |

