Chemistry:Rhodamine B
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
9-(2-Carboxyphenyl)-6-(diethylamino)-N,N-diethyl-3H-xanthen-3-iminium chloride | |
| Other names
Rhodamine 610, C.I. Pigment Violet 1, Basic Violet 10, C.I. 45170
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C28H31ClN2O3 | |
| Molar mass | 479.02 |
| Appearance | Green powder[1] |
| Melting point | 210 to 211 °C (410 to 412 °F; 483 to 484 K) (Decomposes) |
| 8 to 15 g/L (20 °C)[2][nt 1] | |
| Hazards | |
| Safety data sheet | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Rhodamine B /ˈroʊdəmiːn/ is a chemical compound and a dye. It is often used as a tracer dye within water to determine the rate and direction of flow and transport. Rhodamine dyes fluoresce and can thus be detected easily and inexpensively with fluorometers.
Other uses

Rhodamine B is often mixed with herbicides to show where they have been used.[3]
It is also being tested for use as a biomarker in oral rabies vaccines for wildlife, such as raccoons, to identify animals that have eaten a vaccine bait. The rhodamine is incorporated into the animal's whiskers and teeth.[4] Rhodamine B is an important hydrophilic xanthene dye well known for its stability and is widely used in the textile industry, leather, paper printing, paint, coloured glass and plastic industries.[5]
Rhodamine B (BV10) is mixed with quinacridone magenta (PR122) to make the bright pink watercolor known as Opera Rose.[6]
Properties
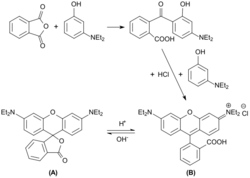
Rhodamine B can exist in equilibrium between two forms: an "open"/fluorescent form and a "closed"/nonfluorescent spirolactone form. The "open" form dominates in acidic condition while the "closed" form is colorless in basic condition.[7]

The fluorescence intensity of rhodamine B will decrease as temperature increases.[8]
The solubility of rhodamine B in water varies by manufacturer, and has been reported as 8 g/L and ~15 g/L,[2] while solubility in alcohol (presumably ethanol) has been reported as 15 g/L.[nt 1] Chlorinated tap water decomposes rhodamine B. Rhodamine B solutions adsorb to plastics and should be kept in glass.[9] Rhodamine B is tunable around 610 nm when used as a laser dye.[10] Its luminescence quantum yield is 0.65 in basic ethanol,[11] 0.49 in ethanol,[12] 1.0,[13] and 0.68 in 94% ethanol.[14] The fluorescence yield is temperature dependent;[15] the compound is fluxional in that its excitability is in thermal equilibrium at room temperature.[16]
Safety and health
In California, rhodamine B is suspected to be carcinogenic and thus products containing it must contain a warning on its label.[17] Cases of economically motivated adulteration, where it has been illegally used to impart a red color to chili powder, have come to the attention of food safety regulators.[18]
See also
References
- ↑ R. W. Ramette; E. B. Sandell (1956). "Rhodamine B Equilibria" (in en). Journal of the American Chemical Society 78 (19): 4872–4878. doi:10.1021/ja01600a017. Bibcode: 1956JAChS..78.4872R.
- ↑ 2.0 2.1 "Safety data sheet". Roth. 2013. https://www.carlroth.com/medias/SDB-T130-AU-EN.pdf?context=bWFzdGVyfHNlY3VyaXR5RGF0YXNoZWV0c3wyMzk1MjZ8YXBwbGljYXRpb24vcGRmfHNlY3VyaXR5RGF0YXNoZWV0cy9oYjEvaDUxLzg5Njk3MDM2ODYxNzQucGRmfDg5ZTFiNGZmNDIzMzc3NDczZjhlYjA3ODBmY2JiOTBkODkyNDc3ZWI3NDU0ODJiNTBmMWM1ZjAzNDI4NDZhMjQ.
- ↑ "Evaluation of five fluorescent dyes and triethyl phosphate as atmospheric tracers of agricultural sprays". Journal of Environmental Science and Health, Part B 32 (6): 969–83. November 1997. doi:10.1080/03601239709373123. Bibcode: 1997JESHB..32..969C.
- ↑ "Oral rabies vaccination in north america: opportunities, complexities, and challenges". PLOS Neglected Tropical Diseases 3 (12): e549. December 2009. doi:10.1371/journal.pntd.0000549. PMID 20027214.
- ↑ Sudarshan, Shanmugam; Bharti, Vidya Shree; Harikrishnan, Sekar; Shukla, Satya Prakash; RathiBhuvaneswari, Govindarajan (2 October 2022). "Eco-toxicological effect of a commercial dye Rhodamine B on freshwater microalgae Chlorella vulgaris". Archives of Microbiology 204 (10): 658. doi:10.1007/s00203-022-03254-5. PMID 36183287. Bibcode: 2022ArMic.204..658S.
- ↑ MacEvoy, Bruce. "Handprint: color making attributes". www.handprint.com. http://www.handprint.com/HP/WCL/waterc.html.
- ↑ "Investigating rhodamine B-labeled peptoids: scopes and limitations of its applications". Biopolymers 96 (5): 694–701. 2011. doi:10.1002/bip.21617. PMID 22180914.
- ↑ "Thermo-optical characterization of fluorescent rhodamine B based temperature-sensitive nanosensors using a CMOS MEMS micro-hotplate". Sensors and Actuators. B, Chemical 192: 126–133. March 2014. doi:10.1016/j.snb.2013.10.042. PMID 25844025. Bibcode: 2014SeAcB.192..126C.
- ↑ Bedmar, Antonio Plata; Araguás, Luís Araguás (2002). Detection and Prevention of Leaks from Dams. Taylor & Francis. ISBN 90-5809-355-7.
- ↑ Prahl, Scott. "Rhodamine B". OMLC. http://omlc.ogi.edu/spectra/PhotochemCAD/html/rhodamineB.html.
- ↑ "Fluorescence quantum yields of some rhodamine dyes". Journal of Luminescence 27 (4): 455–462. 1982. doi:10.1016/0022-2313(82)90045-X. Bibcode: 1982JLum...27..455K. http://habana.qfa.uam.es/~lmc/ref/old/kubin82.pdf. Retrieved 2019-03-22.
- ↑ Casey, Kelly G.; Quitevis, Edward L. (1988). "Effect of solvent polarity on nonradiative processes in xanthene dyes: Rhodamine B in normal alcohols". The Journal of Physical Chemistry 92 (23): 6590–6594. doi:10.1021/j100334a023.
- ↑ "Radiationless Intermolecular Energy Transfer. III. Determination of Phosphorescence Efficiencies". The Journal of Chemical Physics 41 (10): 3042–3045. 1964. doi:10.1063/1.1725672. Bibcode: 1964JChPh..41.3042K.
- ↑ "The photophysics of rhodamine B". Journal of Photochemistry 18 (4): 335–346. 1982. doi:10.1016/0047-2670(82)87023-8.
- ↑ "Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements". The Journal of Physical Chemistry 84 (14): 1871–1872. 1980. doi:10.1021/j100451a030.
- ↑ "Bypassing bleaching with fluxional fluorophores". Nature Methods 16 (5): 357. May 2019. doi:10.1038/s41592-019-0402-2. PMID 31040423.
- ↑ "Naval Jelly MSDS with Rhodamine B". Locite Corporation. 20 October 1998. https://www.brown.edu/Departments/Visual_Art/documents/NavalJelly.pdf.
- ↑ Lin, Shuang (2015). "Rapid and sensitive SERS method for determination of Rhodamine B in chili powder with paper-based substrates". Analytical Methods 7 (12): 5289. doi:10.1039/c5ay00028a. https://www.researchgate.net/publication/278036259. Retrieved 1 February 2018.
Notes
- ↑ 1.0 1.1 Ellis, Roy C. (November 16, 2015). "Reagent and Dye Solubility Chart". IHCWorld. http://www.ihcworld.com/_technical_tips/solubility_chart.htm. "This is to be used as a guide only as solubility data varies between manufacturers for the same product, especially for dyes." Note that most sources simply indicate that the compound is water soluble without providing a g/L value.
 |
