Chemistry:NNK
| |
| Names | |
|---|---|
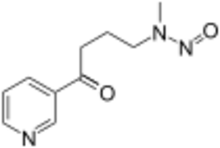
| Preferred IUPAC name
Methyl[4-oxo-4-(pyridin-3-yl)butyl]nitrous amide | |
| Other names
N-Nitrosonornicotine ketone; 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3548355 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| UN number | 2811 |
| |
| |
| Properties | |
| C10H13N3O2 | |
| Molar mass | 207.233 g·mol−1 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H301, H302, H317, H351 | |
| P201, P202, P261, P264, P270, P272, P280, P281, P301+310, P301+312, P302+352, P308+313, P321, P330, P333+313, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nicotine-derived nitrosamine ketone (NNK) is one of the key tobacco-specific nitrosamines derived from nicotine. It plays an important role in carcinogenesis.[1] The conversion of nicotine to NNK entails opening of the pyrrolidine ring.
Synthesis and occurrence
NNK can be produced by standard methods of organic synthesis.[2]
Tobacco
NNK is both found in cured tobacco and is produced during its burning (pyrolysis).[3] The amount of NNK delivered in cigarette smoke ranged from 30 to 280 ng/cigarette in one study[4] and 12 to 110 ng/cigarette in another.[5]
Sun-cured tobaccos (a.k.a. "Oriental") contain very little NNK and other TSNAs due to low-nitrate soil, lack of nitrate fertilizer, and sun-curing. Flue-cured tobacco (a.k.a. "Virginia" tobacco[6]), especially when using an open flame, contains most of the NNK in American blended tobaccos[7] although Marlboro's "virginia blend" had the lowest levels of NNK per nicotine out of many tested with the exception of Natural American Spirit.[8]
e-Cigarettes
e-Cigarette do not convert nicotine to NNK due to their lower operating temperatures.[9] The amount of NNK delivered by e-cigarettes reaches 2.8 ng per 15 puffs (approximately 1 cigarette).[5] NNK was found in 89% of Korean e-cigarette liquids. Concentrations range from 0.22 to 9.84 µg/L.[10] For the product that had the highest amount, if 1 ml is equival to 20 cigarettes,[11] there would be 9.84/20 = 0.5 ng NNK per e-cig cigarette dose. Cigarettes with 1 gram of tobacco average about 350 ng.[7]
Biology
Metabolism
NNK is initially a procarcinogen that needs activation to exert its effects. The activation of NNK is done by enzymes of the cytochrome pigment (CYP) multigene family. These enzymes catalyze hydroxylation reactions. Beside the CYP family NNK can also be activated by metabolic genes, like myeloperoxidase (MPO) and epoxide hydrolase (EPHX1).[citation needed] NNK can be activated by two different routes, the oxidative path and the reductive path. In the oxidative metabolism NNK undergoes an α-hydroxylation catalyzed by cytochrome P450. This reaction can be done by two pathways namely by α-methylhydroxylation or by α-methylenehydroxylation. Both pathways produce the carcinogenic metabolized isoform of NNK, NNAL.[citation needed]
In the reductive metabolism NNK undergoes either a carbonyl reduction or a pyridine N-oxidation, both producing NNAL.[citation needed]
NNAL can be detoxified by glucuronidation producing a non-carcinogenic compounds known as NNAL-Glucs. The glucuronidation can take place on the oxygen next to the ring (NNAL-O-Gluc), or it takes place on the nitrogen inside the ring(NNAL-N-Gluc). The NNAL-Glucs are then excreted by the kidneys into the urine.[12]
Signaling pathways
Once NNK is activated, NNK initiates a cascade of signaling pathways (for example ERK1/2, NFκB, PI3K/Akt, MAPK, FasL, K-ras), resulting in uncontrolled cellular proliferation and tumorigenesis.[1]
NNK activates µ en m-calpain kinase which induce lung metastasis via the ERK1/2 pathway. This pathway upregulate cellular myelocytomatosis (c-Myc) and B cell leukemia/lymphoma 2 (Bcl2) in which the two oncoprotein are involved in cellular proliferation, transformation and apoptosis. Also does NNK promotes cell survival via phosphorylation with cooperation of c-Myc and Bcl2 causing cellular migration, invasion and uncontrolled proliferation.[13]
The ERK1/2 pathway also phosphorylate NFκB causing an upregulation of cyclin D1, a G1 phase regulator protein. When NNK is present it directly involves cellular survival dependent on NFκB. Further studies are needed to better understand NNK cellular pathways of NFκB.[14][15]
The phosphoinositide 3-kinase (PI3K/Akt) pathway is also an important contributor to NNK-induced cellular transformations and metastasis. This process ensures the proliferation and survival of tumorigenic cells.[16] The ERK1/2 and Akt pathways show consequential changes in levels of protein expression as a result of NNK-activation in the cells, but further research is needed to fully understand the mechanism of NNK-activated pathways.[citation needed]
Pathology
Toxicity
NNK is known as a mutagen, which means it causes polymorphisms in the human genome. Studies showed that NNK induced gene polymorphisms in cells that involve in cell growth, proliferation and differentiation. There are multiple NNK dependent routes that involve cell proliferation. One example is the cell route that coordinates the downregulation of retinoic acid receptor beta (RAR-β). Studies showed that with a 100 mg/kg dose of NNK, several point mutations were formed in the RAR-β gene, inducing tumorigenesis in the lungs.[citation needed]
Other genes affected by NNK include sulfotransferase 1A1 (SULT1A1), transforming growth factor beta (TGF-β), and angiotensin II (AT2).[citation needed]
NNK plays a very important role in gene silencing, modification, and functional disruption which induce carcinogenesis.[1]
Inhibition
Chemical compounds derived from cruciferous vegetables and EGCG inhibit lung tumorigenesis by NNK in animal models.[17] Whether these effects have any relevance to human health is unknown and is a subject of ongoing research.[citation needed]
See also
- Toxification
References
- ↑ 1.0 1.1 1.2 Akopyan, Gohar; Bonavida, Benjamin (2006). "Understanding tobacco smoke carcinogen NNK and lung tumorigenesis". International Journal of Oncology 29 (4): 745–52. doi:10.3892/ijo.29.4.745. PMID 16964372.
- ↑ Castonguay, Andre; Hecht, Stephen S. (1985). "Synthesis of Carbon-14 Labeled 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone". Journal of Labelled Compounds and Radiopharmaceuticals 22: 23–8. doi:10.1002/jlcr.2580220104.
- ↑ Adams, John D.; Lee, Suk Jong; Vinchkoski, Norma; Castonguay, Andre; Hoffmann, Dietrich (1983). "On the formation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone during smoking". Cancer Letters 17 (3): 339–46. doi:10.1016/0304-3835(83)90173-8. PMID 6831390.
- ↑ Djordjevic, M. V.; Stellman, S. D.; Zang, E (2000). "Doses of Nicotine and Lung Carcinogens Delivered to Cigarette Smokers". Journal of the National Cancer Institute 92 (2): 106–11. doi:10.1093/jnci/92.2.106. PMID 10639511.
- ↑ 5.0 5.1 Grana, R.; Benowitz, N.; Glantz, S. A. (2014). "E-Cigarettes: A Scientific Review". Circulation 129 (19): 1972–86. doi:10.1161/CIRCULATIONAHA.114.007667. PMID 24821826.
- ↑ "Tobacco farming". http://www.pmi.com/eng/our_products/pages/about_tobacco.aspx.
- ↑ 7.0 7.1 Gunduz, I.; Kondylis, A.; Jaccard, G.; Renaud, J.-M.; Hofer, R.; Ruffieux, L.; Gadani, F. (2016). "Tobacco-specific N-nitrosamines NNN and NNK levels in cigarette brands between 2000 and 2014". Regulatory Toxicology and Pharmacology 76: 113–20. doi:10.1016/j.yrtph.2016.01.012. PMID 26806560.
- ↑ Appleton, Scott; Olegario, Raquel M.; Lipowicz, Peter J. (2013). "TSNA levels in machine-generated mainstream cigarette smoke: 35years of data". Regulatory Toxicology and Pharmacology 66 (2): 197–207. doi:10.1016/j.yrtph.2013.03.013. PMID 23557986.
- ↑ Farsalinos, Konstantinos; Gillman, Gene; Poulas, Konstantinos; Voudris, Vassilis (2015). "Tobacco-Specific Nitrosamines in Electronic Cigarettes: Comparison between Liquid and Aerosol Levels". International Journal of Environmental Research and Public Health 12 (8): 9046–53. doi:10.3390/ijerph120809046. PMID 26264016.
- ↑ Kim, Hyun-Ji; Shin, Ho-Sang (2013). "Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography–tandem mass spectrometry". Journal of Chromatography A 1291: 48–55. doi:10.1016/j.chroma.2013.03.035. PMID 23602640.
- ↑ "VapeMail Ban - Brands Still Shipping in 2021". http://www.electroniccigaretteconsumerreviews.com/how-much-nicotine-is-in-one-cigarette/.
- ↑ Wiener, D.; Doerge, D. R.; Fang, J. L.; Upadhyaya, P.; Lazarus, P (2004). "Characterization of N-glucuronidation of the lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol (NNAL) in human liver: importance of UDP-glucuronosyltransferase 1A4". Drug Metabolism and Disposition 32 (1): 72–9. doi:10.1124/dmd.32.1.72. PMID 14709623.
- ↑ Jin, Z.; Gao, F.; Flagg, T.; Deng, X. (2004). "Tobacco-specific Nitrosamine 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone Promotes Functional Cooperation of Bcl2 and c-Myc through Phosphorylation in Regulating Cell Survival and Proliferation". Journal of Biological Chemistry 279 (38): 40209–19. doi:10.1074/jbc.M404056200. PMID 15210690.
- ↑ Ho, Y; Chen, C; Wang, Y; Pestell, R; Albanese, C; Chen, R; Chang, M; Jeng, J et al. (2005). "Tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) induces cell proliferation in normal human bronchial epithelial cells through NFκB activation and cyclin D1 up-regulation". Toxicology and Applied Pharmacology 205 (2): 133–48. doi:10.1016/j.taap.2004.09.019. PMID 15893541.
- ↑ Tsurutani, J.; Castillo, S. S.; Brognard, J.; Granville, C. A.; Zhang, C; Gills, J. J.; Sayyah, J.; Dennis, P. A. (2005). "Tobacco components stimulate Akt-dependent proliferation and NFkappaB-dependent survival in lung cancer cells". Carcinogenesis 26 (7): 1182–95. doi:10.1093/carcin/bgi072. PMID 15790591.
- ↑ West, K. A.; Linnoila, I. R.; Belinsky, S. A.; Harris, C. C.; Dennis, P. A. (2004). "Tobacco carcinogen-induced cellular transformation increases activation of the phosphatidylinositol 3'-kinase/Akt pathway in vitro and in vivo". Cancer Research 64 (2): 446–51. doi:10.1158/0008-5472.CAN-03-3241. PMID 14744754.
- ↑ Chung, F.-L.; Morse, M. A.; Eklind, K. I.; Xu, Y. (1993). "Inhibition of the Tobacco-Specific Nitrosamine-Induced Lung Tumorigenesis by Compounds Derived from Cruciferous Vegetables and Green Tea". Annals of the New York Academy of Sciences 686 (1): 186–201; discussion 201–2. doi:10.1111/j.1749-6632.1993.tb39174.x. PMID 8512247. Bibcode: 1993NYASA.686..186C.
External links
 |

