Chemistry:Casuarictin
From HandWiki
| |
| Names | |
|---|---|
| Systematic IUPAC name
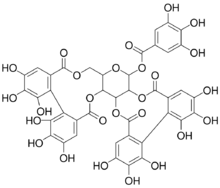
(10aR,11S,12aR,25aR,25bS)-2,3,4,5,6,7,17,18,19,20,21,22-Dodecahydroxy-9,15,24,27-tetraoxo-9,10a,11,12a,13,15,24,25a,25b,27-decahydrodibenzo[g,i]dibenzo[6′,7′:8′,9′][1,4]dioxecino[2′,3′:4,5]pyrano[3,2-b][1,5]dioxacycloundecin-11-yl 3,4,5-trihydroxybenzoate | |
| Other names
Sanguiin H 11
Sanguiin H-11 1(β)-O-Galloylpedunculagin | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C41H28O26 | |
| Molar mass | 936.64 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Casuarictin is an ellagitannin, a type of hydrolysable tannin. It can be found in Casuarina and Stachyurus species.[1]
It is formed from two hexahydroxydiphenic acid units and one gallic acid unit linked to a glucose molecule.
The molecule is formed from tellimagrandin II, itself formed from pentagalloyl glucose via oxidation. Casuarictin is transformed into pedunculagin via loss of a gallate group, and further into castalagin via glucose pyranose ring opening.
Oligomers
Sanguiin H-6 is a dimer, Lambertianin C is trimer and lambertianin D is a tetramer of casuarictin.
References
 |

