Chemistry:Lipstatin
| |
| Names | |
|---|---|
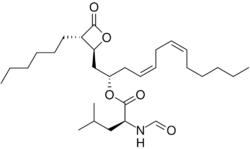
| Systematic IUPAC name
(2S,4Z,7Z)-1-[(2S,3S)-3-Hexyl-4-oxooxetan-2-yl]trideca-4,7-dien-2-yl (2S)-2-formamido-4-methylpentanoate | |
| Other names
(2S,4Z,7Z)-1-[(2S,3S)-3-Hexyl-4-oxo-2-oxetanyl]-4,7-tridecadien-2-yl N-formyl-L-leucinate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| MeSH | Lipstatin |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C29H49NO5 | |
| Molar mass | 491.713 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Lipstatin is a potent, irreversible inhibitor of pancreatic lipase. It is a natural product that was first isolated from the actinobacterium Streptomyces toxytricini.[1]
The popular antiobesity drug orlistat (trade names Xenical and alli) is a saturated derivative of lipstatin.
Biosynthesis
Lipstatin is composed of a 2-hexyl-3,5-dihydroxy-7,10-hexadecadienoic-β-lactone 22 carbon backbone from fatty acid synthesis pathway and an N-formyl-L-leucine group ester linked to the 5-hydroxyl group of the back bone. The composts of the lipstatin are ultimately from linoleic acid, octanoic acid, and L-leucine.[2]
The 22 carbon β-lactone moiety is derived from Claisen condensation between 3-hydroxytetradeca-5,8-dienyl-CoA derived from linoleic acid and hexyl-malonyl-ACP derived from octanoic acid.[2] [3][4]Linoleic acid is first attached to CoA through some acyl-CoA synthetase homologue synthesized by lipstatin biosynthetic operons (Lst) and goes through two β-oxidation to hydroxytetradeca-5,8-dienyl-CoA. 3’ hydroxyl group from solution H2O is added by enoyl reductase homologue followed by enoyl hydratase homologue.[5] Octanoic acid is also attached to CoA through similar acyl-CoA synthetase homologue (LstC) to form octanoyl-CoA. Octanoyl-CoA is 2’ carboxylated and loaded to acyl carrier protein (ACP) borrowed from primary metabolism to form hexyl-malonyl-ACP.[4]
The β-lactone ring is formed by reduction of 3-keto group by 3-hydroxysteroid dehydrogenase homologue followed by a spontaneously nucleophilic attack of the 3-hydroxyl group on the carbonyl of the ACP-tether acyl intermediate. This step follows the esterification of the N-formyl-L-leucine group.[2]
The N-formyl-L-leucine group is derived from L-leucine. L-leucine was activated by LstE forming thioester and its α-amine group is formylated by LstF. Finally, 5-hydroxyl of 22 carbon β-lactone backbone nucleophilic attacks on the acyl carbon of the formyl-leucine and forms the lipstatin.[2]
See also
References
- ↑ "Lipstatin, an inhibitor of pancreatic lipase, produced by Streptomyces toxytricini. I. Producing organism, fermentation, isolation and biological activity". J Antibiot (Tokyo) 40 (8): 1081–5. 1987. doi:10.7164/antibiotics.40.1081. PMID 3680018.
- ↑ 2.0 2.1 2.2 2.3 Bai, Tingli; Zhang, Daozhong; Lin, Shuangjun; Long, Qingshan; Wang, Yemin; Ou, Hongyu; Kang, Qianjin; Deng, Zixin et al. (2014-12-15). "Operon for Biosynthesis of Lipstatin, the Beta-Lactone Inhibitor of Human Pancreatic Lipase" (in en). Applied and Environmental Microbiology 80 (24): 7473–7483. doi:10.1128/AEM.01765-14. ISSN 0099-2240. PMID 25239907.
- ↑ Goese, Markus; Eisenreich, Wolfgang; Kupfer, Ernst; Stohler, Peter; Weber, Wolfgang; Leuenberger, Hans G.; Bacher, Adelbert (2001-06-01). "Biosynthesis of Lipstatin. Incorporation of Multiply Deuterium-Labeled (5Z,8Z)-Tetradeca-5,8-dienoic Acid and Octanoic Acid". The Journal of Organic Chemistry 66 (13): 4673–4678. doi:10.1021/jo010230b. ISSN 0022-3263.
- ↑ 4.0 4.1 Demirev, Atanas V.; Khanal, Anamika; Sedai, Bhishma R.; Lim, Si Kyu; Na, Min Kyun; Nam, Doo Hyun (2010-05-02). "The role of acyl-coenzyme A carboxylase complex in lipstatin biosynthesis of Streptomyces toxytricini" (in en). Applied Microbiology and Biotechnology 87 (3): 1129–1139. doi:10.1007/s00253-010-2587-2. ISSN 0175-7598. PMID 20437235.
- ↑ Goese, Markus; Eisenreich, Wolfgang; Kupfer, Ernst; Weber, Wolfgang; Bacher, Adelbert (2000-07-14). "Biosynthetic Origin of Hydrogen Atoms in the Lipase Inhibitor Lipstatin" (in en). Journal of Biological Chemistry 275 (28): 21192–21196. doi:10.1074/jbc.M003094200. ISSN 0021-9258. PMID 10801870.
 |


