Chemistry:Chloryl
| |
| |
| Names | |
|---|---|
| IUPAC name
Dioxo-λ3-chloranium
| |
| Other names
Chlorylium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 100601 | |
| |
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
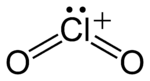
In chemistry, chloryl refers to a triatomic cation with chemical formula ClO+2. This species has the same general structure as chlorite (ClO−2) but it is electronically different, with chlorine having a +5 oxidation state (rather than the +3 of chlorite). This makes it a rare example of a positively charged oxychloride. Chloryl compounds, such as FClO2 and [ClO2][RuF6], are all highly reactive and react violently with water and most organic compounds.[1][2]
Structure
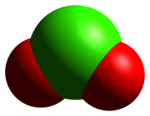
The ClO+2 cation is isoelectronic with SO2,[3] and has a bent structure with a bond angle close to 120°. The Cl–O bond is of bond order 1.5, with its Lewis structure consisting of a double bond and a dative bond which does not utilize d-orbitals.[4]
The red color of ClO+2 is caused by electron transitions into an antibonding orbital. The analogous transition in SO2 is not in the visible spectrum, so SO2 is colorless. The strength of interaction with the counterion affects the energy of this antibonding orbital; thus, in colorless chloryl compounds, strong interactions with the counterion, corresponding with the higher covalent character of the bonding, shift the transition energy out of the visible spectrum.[3]
Compounds

There are two categories of chloryl compounds. The first category is colorless, and includes chloryl fluoride (FClO2). These are moderately reactive. Although named as an ionic "chloryl" compound, chloryl fluoride is more a covalent compound than an ionic compound of fluoride and chloryl cation.
The second category features red-colored compounds that are highly reactive. These include chloryl fluorosulfate, ClO2SO3F, and dichloryl trisulfate, (ClO2)2(S3O10). These chloryl compounds form red solutions in fluorosulfuric acid, and do contain a red-colored ClO+2 cation which dissociates in solution. In the solid state, the Raman and infrared spectra indicate strong interactions with the counterion.[3][1] Not all chloryl compounds in the solid state are necessarily ionic. The reaction products of FClO2 with BF3 and PF5 are assumed to be molecular adducts rather than true salts.[3][5]
One notable chloryl compound is dichlorine hexoxide, which exists as an ionic compound more accurately described as chloryl perchlorate, [ClO2]+[ClO4]−.[6] It is a red fuming liquid under standard conditions.
Chloryl compounds are best prepared by the reaction of FClO2 with a strong Lewis acid. For example:[5]
- FClO2 + AsF5 → [ClO2][AsF6]
Other synthesis routes are also possible, including:[5]
- 5 ClO2 + 3 AsF5 → 2 [ClO2][AsF6] + AsF3O + 4 Cl2
- Cl2O6 + 2 SbF5 → [ClO2][SbF6] + SbF3O + FClO3
Metathesis reactions may be carried out with strong Lewis bases. For example, the reaction of the hexafluoroplatinate salt with nitryl fluoride yields the nitronium salt:[5]
- [ClO2][PtF6] + FNO2 → [NO2][PtF6] + FClO2
References
- ↑ 1.0 1.1 Christe, K. O.; Schack, C. J.; Pilipovich, D.; Sawodny, W. (1969). "Chloryl cation, ClO+2". Inorganic Chemistry 8 (11): 2489–2494. doi:10.1021/ic50081a050.
- ↑ Bougon, R.; Cicha, W. V.; Lance, M.; Meublat, L.; Nierlich, M.; Vigner, J. (1991). "Preparation characterization and crystal structure of chloryl hexafluororuthenate(1-). Crystal structure of [ClF2]+[RuF6]−". Inorganic Chemistry 30 (1): 102–109. doi:10.1021/ic00001a019.
- ↑ 3.0 3.1 3.2 3.3 Carter, H. A.; Johnson, W. M.; Aubke, F. (15 December 1969). "Chloryl compounds. Part II. Chloryl hexafluoroarsenate and chloryl fluoride". Canadian Journal of Chemistry 47 (24): 4619–4625. doi:10.1139/v69-763.
- ↑ David L. Cooper (2001). "Spin-coupled description of the chemical bonding to hypercoordinate chlorine". Theoretical Chemistry Accounts 105 (4–5): 323–327. doi:10.1007/PL00013292.
- ↑ 5.0 5.1 5.2 5.3 K. O. Christe; C. J. Schack (1976). Harry Julius Emeléus, A. G. Sharpe. ed. Chlorine Oxyfluorides. Advances in inorganic chemistry and radiochemistry, Volume 18. Academic Press. pp. 356–358. ISBN 0-12-023618-4. https://books.google.com/books?id=EWlBFTxYth4C&q=chloryl+cation&pg=PA319.
- ↑ Tobias, K. M.; Jansen, M. (1986). "Crystal Structure of Cl2O6". Angewandte Chemie International Edition in English 25 (11): 993–994. doi:10.1002/anie.198609931.
External links
 |

