Chemistry:4-Ethylphenol
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Ethylphenol[2] | |
| Other names
p-Ethylphenol
1-Ethyl-4-hydroxybenzene 1-Hydroxy-4-ethylbenzene 4-Hydroxyphenylethane | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.167 g·mol−1 |
| Appearance | White solid |
| Odor | leather-like |
| Melting point | 45.1 °C (113.2 °F; 318.2 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 104 °C (219 °F; 377 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethylphenol (4-EP) is an organic compound with the formula C2H5C6H4OH. It is one of three isomeric ethylphenols. A white solid, it occurs as an impurity in xylenols and as such is used in the production of some commercial phenolic resins. It is also a precursor to 4-vinylphenol.[3]
Natural occurrences
In wine and beer, 4-EP is produced by the yeast Brettanomyces. At concentrations greater than 140 μg/L (typical sensory threshold) it gives the wine aromas described as barnyard, medicinal, band-aids, and mousy. In certain Belgian beer styles, a high 4-EP level may be desirable; however, very high levels of the compound in wine can render it undrinkable. The level of 4-EP is roughly proportional to Brettanomyces concentration and activity, and can therefore be used as an indicator of the yeast's presence. There are significant differences between strains of Brettanomyces in their ability to produce 4-EP.
4-EP is also a component of castoreum, the exudate from the castor sacs of the mature North American beaver (Castor canadensis) and the Eurasian beaver (Castor fiber), used in perfumery.
Biochemistry
4-EP is biosynthesized in two steps from p-coumaric acid. Decarboxylation gives 4-vinylphenol as catalyzed by the enzyme cinnamate decarboxylase.[4] 4-Vinylphenol is further reduced to 4-ethylphenol by the enzyme vinyl phenol reductase. Coumaric acid is sometimes added to microbiological media, enabling the positive identification of Brettanomyces by smell.
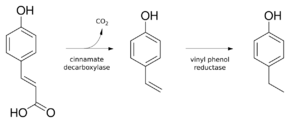 The conversion of p-coumaric acid to 4-EP by Brettanomyces
The conversion of p-coumaric acid to 4-EP by Brettanomyces
See also
- 4-Ethylguaiacol
- Yeast in winemaking
- Wine fault
- Wine chemistry
References
- ↑ "4-Ethylphenol MSDS". http://physchem.ox.ac.uk/MSDS/ET/4-ethylphenol.html.
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4. "Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p."
- ↑ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
- ↑ Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol at etslabs.com
External links
 |


