Introduction to the metric system

The metric system was developed during the French Revolution to replace the various measures previously used in France. The metre (spelled "meter" in American English) is the unit of length in the metric system and was originally based on the dimensions of the earth, as far as it could be measured at the time. The litre (or in American English "liter"), is the unit of volume and was defined as one thousandth of a cubic metre. The metric unit of mass is the kilogram and it was defined as the mass of one litre of water. The metric system was, in the words of French philosopher Marquis de Condorcet, "for all people for all time".[1]
The metric system has names to cover different ranges of the same measure. Instead of using names based on the context of the measure, the metric system mainly uses names made by adding prefixes, such as kilo- or milli-, as decimal multipliers to the base unit names. Thus, one kilogram is 1000 grams and one kilometre is 1000 metres.
During the nineteenth century the metric system was adopted by both the worldwide scientific community and many countries as the system of measurement. It therefore became truly international. Until 1875 the French government owned the prototype metre and kilogram, but in that year the Convention of the metre was signed and control of the standards relating to mass and length passed on to a trio of inter-government organisations.
In 1960 the metric system was extensively revised to form the International System of Units, abbreviated to "SI".
Origins
On the eve of the French Revolution , France had an estimated quarter of a million different units of measurement. In many cases the value of a unit differed from town to town and even from trade to trade even though they might have the same name. While certain standards, such as the pied du roi (the King's foot) had a degree of pre-eminence and were used by savants (scientists), many traders used their own measuring devices. This gave scope for fraud and hindered commerce and industry.[2] The metric system was designed to replace this confusion with a radical new system with fixed values.[1]
In France[2] and the rest of Europe there was a multitude of measurement units. The differences were like those between United States customary units and United Kingdom imperial units of liquid volume measures – a US pint consists of 16 US fluid ounces while an imperial pint is 20 UK fluid ounces and the US fluid ounce is about 4% larger than the UK fluid ounce. Differences such as these occurred across Europe.
Between 1790 and 1800, during the French Revolution , and with the backing of Louis XVI[after his death in 1793??], the system of weights and measures was totally reformed.[3] The new system of measures had a rational mathematical basis and was part of the radical effort to sweep away old traditions and conventions and replace them with something new and better.[4] The French philosopher, the Marquis de Condorcet, who was one of those entrusted by Louis XVI to overhaul the system of measurement, characterised the metric system as "for all people for all time".[5]
The key units of the republican measures system were:
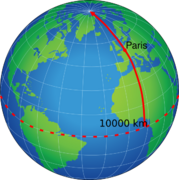
- The mètre – the unit of length, defined as one ten-millionth of the distance between the north pole and the equator on the meridian passing through Paris[6]
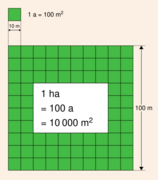
- The are – for land area, defined as the area of a square with sides of length 10 metres
- The stère – for volume (particularly of stacked firewood),[7] defined as 1 cubic metre
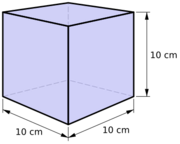
- The litre – for dry and liquid volume, defined as the volume of a cube with sides of one-tenth of a metre
- The gramme – for weight, defined as the weight of a cube of pure water with sides of one-hundredth of a metre and at the temperature of melting ice.
Since it was not practical to determine the metre and the kilogram with adequate precision and repeatability, it was decided to use artefacts as the reference kilogram and metre, against which instruments could be calibrated. The mètre des Archives and kilogramme des Archives were manufactured to meet these definitions as closely as possible. The definition of the metre has since been revised to be independent of any artifact,[8] and a similar redefinition of the kilogram occurred in May 2019.
The new system was not popular and people continued to use their customary measures. Napoleon recognised the value of a sound basis for a system of measurement but ridiculed the metric system. In 1812 he introduced the mesures usuelles, a modification of the metric measures for use in small retail businesses. These mesures usuelles used some older unit names but used the metre des Archives and the kilogramme des Archives as its basis for measurement. However, all government, legal and similar works still had to use the metric system and the metric system continued to be taught at all levels of education.[9] This system survived in France until the metric system was reinstated for all purposes in 1840.
International metric system
The metric system developed as the understanding of science and in measuring techniques have advanced. In 1875, the Convention of the metre was signed and control of the metric system passed from France to a trio of inter-government organisations headed by the Conférence générale des poids et mesures (CGPM) and based in Sèvres, France.[10] In 1960, at the 11th conference of the CGPM, the metric system was overhauled and the resultant system named "The International System of Units", (also known as "SI", an abbreviation Système international d'unités).[11]
In this article, the term "SI" will be used to describe items that are specific to post-1960 developments, otherwise the term "metric system" will be used.
| SI units in everyday use | ||
|---|---|---|
| Quantity | Unit | Symbol |
| Length | metre | m |
| Mass | kilogram | kg |
| Time | second | s |
| Temperature | degree Celsius |
°C |
| Potential difference |
volt | V |
| Energy | joule | J |
| Power | watt | W |
| Pressure | pascal | Pa |
The driving force behind the metric system was the need for a single, rational and universal system of weights and measures that could be used worldwide.
Units
The names of the units of measure used in the metric system consist of two parts: a unit name (for example "metre", "gram", "litre") and an associated multiplier prefix (for example "milli-" meaning 1⁄1000, "kilo-" meaning 1000). The result is that there are a variety of different named units available to measure the same quantity (for example 10 millimetres = 1 centimetre, 100 centimetres = 1 metre, 1000 metres = 1 kilometre). Each unit and each prefix has a standard symbol (not abbreviation) associated with it.
In 1861, during discussions about standardising electrical units of measure, Charles Bright and Latimer Clark proposed that these units be named, not in relation to what they are used for, or common objects, but after eminent scientists; with the electrical units of resistance, potential difference and capacitance being named the ohm, volt and farad in honour of Georg Ohm, Alessandro Volta and Michael Faraday respectively. This proposal had the support of William Thomson (Lord Kelvin)[12] who had been instrumental in forming the Committee of Electrical Standards of the British Association for the Advancement of Science. The use of scientists' names for such units was subsequently extended to other units, including the watt named after James Watt and the degree Celsius named after Anders Celsius.
Prefixes
| Metric prefixes in everyday use | |||
|---|---|---|---|
| Text | Symbol | Factor | Power |
| tera | T | 1000000000000 | 1012 |
| giga | G | 1000000000 | 109 |
| mega | M | 1000000 | 106 |
| kilo | k | 1000 | 103 |
| hecto | h | 100 | 102 |
| deca | da | 10 | 101 |
| (none) | (none) | 1 | 100 |
| deci | d | 0.1 | 10−1 |
| centi | c | 0.01 | 10−2 |
| milli | m | 0.001 | 10−3 |
| micro | μ | 0.000001 | 10−6 |
| nano | n | 0.000000001 | 10−9 |
| pico | p | 0.000000000001 | 10−12 |
Historically, individual units evolved which were based on the size and context of what was being measured. These units could avoid the need to use large numbers of smaller units or small numbers of larger units for a measurement. These units were generally defined as a convenient multiple of a smaller unit or a convenient division of a larger unit. Thus, in pre-revolutionary France, the inch was divided into 12 lines and each line was subdivided into 12 points.[13] Similarly, astronomers introduced the light-year to describe large distances. The metric system on the other hand uses prefixes to denote multipliers of the one basic unit. For example, the prefix "kilo" is used to denote a multiplier of 1000; thus one kilometre is 1000 metres, one kilogram is 1000 grams, and one kilowatt is 1000 watts.
SI symbols
Each unit and each prefix in the metric systems has been allocated a unique symbol by the CGPM. Unlike abbreviations which are a contraction of the local word for the unit in question, and which can therefore differ from one language to another, SI symbols are a form of standardised mathematical notation to represent the units and are the same in any language (compare chemical symbols).
There are certain circumstances where abbreviations (as opposed to the SI symbols) are used, particularly where safety is concerned. One such instance is the use of "mcg" rather than "μg" to represent "micrograms" in the pharmaceutical industry.[14]
Units
| Non-SI everyday units acceptable for use with SI | ||
|---|---|---|
| Quantity | Unit | Symbol |
| Time | day (86400 s) hour (3600 s) minute (60 s) |
d h min |
| Plane angle | degree ((π/180) rad) | ° |
| Mass | tonne (1000 kg) | t |
| Volume | litre (0.001 m3) | L or l |
| Area | hectare (10,000 m2) | ha |
| Pressure | bar (100 kPa) | bar |
There are three basic classes of units in SI:
- Base units such as the metre, kilogram, second.[15] Base units are defined with great precision as they form the basis for all other metric units of measure.
- Derived units which are formed by operating on base units or combinations of base units and other derived units.[16] For example, a C♯ tuning fork vibrates 261.6 times per second and therefore has a frequency of 261.6 hertz. In this instance "hertz" is a derived unit as it is automatically defined from the definition of the second. Other derived units might be built up from one or more base units.
- Non-SI units accepted for use within SI such as litre, hectare and tonne. The CGPM (the guardians of the SI) have recognised that numerous units of measure are used sufficiently widely and consistently around the globe that they warrant official recognition. Other such units are "minute" and "hour".[17] The CGPM have catalogued such units of measure and given them unique symbols to ensure that they are accorded the same consistency as are SI units. Thus, "kilometres per hour" is always written "km/h" even if the local word for "hour" does not start with an "h"; for example "hour" is written "uur" in Dutch, but Dutch speedometers show "km/h".[18]
This article will not differentiate between these various classes of units, other than to make references to them as appropriate.
Length
The metre is the base unit of length. Its name was derived from the Greek μέτρον καθολικόν (métron katholikón), "a universal measure". This word gave rise to the French mètre which was subsequently introduced into the English language.[19]
The metre is defined as the length of the path travelled by light in a vacuum in 1/299792458 of a second
Originally the metre was to have been one ten millionth of the distance between the North Pole and the equator. The French Academy of Sciences commissioned an expedition led by Jean Baptiste Joseph Delambre and Pierre Méchain, lasting from 1792 to 1799, which measured the distance between the Dunkerque belfry and Montjuïc castle, Barcelona to estimate the length of the meridian arc through Dunkerque (assumed to be the same length as the Paris meridian). This portion of the meridian was to serve as the basis for the length of the half meridian, connecting the North Pole with the equator.[20] In 1799 a metre bar was manufactured based on results of this survey. Although the bar was subsequently found to be 0.02% shorter than it should have been, the metre has always been based on the length of the bar rather than the half meridian.
| Metric Unit |
Imperial/ Customary Equivalent |
Visualisation |
|---|---|---|
| 1 km | 0.62 miles 1100 yd |
The Mall (links Trafalgar Square and Buckingham Palace) Niagara Falls (Bank to bank) |
| 100 m | 110 yd | Length of a gridiron football (Canadian), association football (soccer) or rugby field Length of four-coach train |
| 10 m | 33 ft | Width of a tennis court (10.97 m) |
| 1 m 100 cm |
1.1 yd 3.3 ft 40 in |
Length of a baseball bat (maximum = 1.067 m) Length of a cricket bat (maximum = 0.965 m) |
| 10 cm | 4 in | Width of a man's palm |
| 10 mm 1 cm |
2⁄5 in | Width of an average acorn |
| 1 mm | 0.04 in | Thickness of denim cloth[21] |
| 100 μm | 0.004 in | Thickness of a sheet of photo-copier paper |
| 10 μm | 0.0004 in | Thickness of plastic cling wrap |
Area
The SI unit of area is the square metre (m2), but when the metric system was first introduced in 1795, the unit of land measure was defined as the are, being 100 m2 (or the area equivalent to that of a square having sides of 10 m). This measure was only used in a few countries, but the hectare (100 ares or 10,000 m2), is a non-SI unit that has been catalogued as being acceptable for use with the SI and is in widespread use throughout the world. (A hectare is about 2.5 acres.)
Volume
The SI unit of volume is the cubic metre (m3) – the volume equivalent to the space occupied by a cube with sides of one metre. However, the litre, one of the oldest metric units, having been formally defined in 1795 as the volume occupied by a cube with sides of one tenth of a metre[4] (making it equal to 0.001 m3, or 1 dm3) is in widespread use. The litre is not technically part of SI, but its use is sufficiently widespread that it is "accepted for use within SI".[17]
Mass
SI distinguishes between mass and weight – mass being a measure of the amount of material contained in an object and weight the gravitational force on that object. We normally "weigh" objects by comparing the gravitational force on that object with the gravitational force on an object of known mass (such as a 1 kg "weight"). Although this concept was understood by ancient scientists (Archimedes' principle is based on it), the wording was only formalised in 1901.
The SI base unit of mass is the kilogram which is defined in terms of three fundamental physical constants: The speed of light c, a specific atomic transition frequency ΔνCs, and the Planck constant h. The formal definition is:
The kilogram, symbol kg, is the SI unit of mass. It is defined by taking the fixed numerical value of the Planck constant h to be 6.62607015×10−34 when expressed in the unit J⋅s, which is equal to kg⋅m2⋅s−1, where the metre and the second are defined in terms of c and ΔνCs.
Originally it was defined in 1795 as the mass of one litre of water at the temperature of melting ice (0 °C),[22] though to ensure greater consistency of kilogram artefacts, and to create a practical physical realisation of the kilogram, a platinum artefact intended to have the mass of precisely 1 kg was manufactured and placed in the French Archives in 1799. This artefact was replaced by one of British manufacture in 1889 which was the definitive kilogram until May 2019.
The kilogram is dissimilar to the other SI base units in that it is expressed as a multiple of another unit (the gram) with a multiplier prefix ("kilo") added to it. A teaspoon holds about 5 grams of sugar which makes milligrams or in some case micrograms convenient units to measure medicine doses when they are dispensed in capsules. The ton, variously defined, had long been a customary unit of measure for large masses and in the mid-nineteenth century the metric ton (or tonne) of 1000 kg (i.e. equivalent to the megagram) was introduced. Although the tonne is not an SI Unit, its continued use in many countries has led to it being "accepted for use within SI".[17]
| Metric Unit |
Imperial/ Customary Equivalent |
Visualisation |
|---|---|---|
| 100 Gg (100,000 tonnes) |
98,000 long tons (UK) 110,000 short tons (US) |
Washington Monument in Washington, DC 82,400 tonnes (81,100 long tons; 90,800 short tons), half above ground |
| 10 Gg (10,000 tonnes) |
9,800 long tons 11,000 short tons |
Power shovel Marion 6360 weighs 11,500 tonnes (11,300 long tons; 12,700 short tons) |
| 1,000 Mg, 1 Gg (1,000 tonnes) |
980 long tons 1100 short tons |
Large mining excavator Caterpillar 6090 FS weighs 980 tonnes (960 long tons; 1,080 short tons) |
| 100,000 kg, 100 Mg (100 tonnes) |
98 long tons 110 short tons |
Australian triple road train Gross weight of a triple is 115 tonnes (113 long tons; 127 short tons) |
| 10,000 kg, 10 Mg (10 tonnes) |
22,000 lb 9.8 long tons 11 short tons |
Fully laden box truck; small lorry |
| 1,000 kg, 1 Mg (1 tonne, 1 metric ton) |
2200 lb 0.98 long tons 1.1 short tons |
Small motor car – typically powered by an engine of between 1.0 and 1.2 L |
| 100 kg | 15 stone 11 lb (UK) 220 lb (US) |
Large man – about 15% of US Caucasian males exceed 100 kg[23] |
| 10 kg | 22 lb | Average weight of a 12-month-old child[24] |
| 1 kg | 2.2 lb; 2 lb 3 oz | One litre drink (excluding the weight of the container) |
| 100 g | 31⁄2 oz | Midway between a tennis ball (≈58 g) and a cricket ball (≈160 g) or a baseball (≈145 g) |
| 10 g | 3⁄8 oz | A large coin
|
| 1 g | 15 grains | Three standard paperclips (1.1 g); a plastic pen cap; two peanut seeds[26] |
| 100 mg | 1.5 grains | Low dose (81 mg) enteric coated aspirin tablet – 120 mg with binders |
| 10 mg | 0.15 grains | One third of a standard paper staple |
Temperature
The degree Celsius (symbol: °C) came into use in its present form in 1744 when 0 °C was defined as the freezing point of water and 100 °C was defined as the boiling point of water, both at a pressure of one standard atmosphere.
Before 1948 the unit was known as "centigrade" from the Latin "centum" translated as 100 and "gradus" translated as "steps". However, in France and Spain, the word "centigrade" also meant 0.0001 of a right angle. To avoid confusion, the BIPM and other standards first referred to the degree centigrade as the "centesimal degree" but in 1948, the CGPM changed the name to "degree Celsius", in honour of the Swedish scientist Anders Celsius who first proposed a similar scale (though Celsius' scale had 0 and 100 switched around). However, they retained the symbol °C.
| Temperature point | Metric | Imperial/ Customary |
|---|---|---|
| Sublimation point of dry ice (frozen CO2) at standard atmospheric pressure | −78 °C | –108 °F |
| Melting point of ice | 0 °C | 32 °F |
| Normal human body temperature | 37.0 °C | 98.6 °F |
| Boiling point of water at standard atmospheric pressure | 100 °C | 212 °F |
Time
When the metric system was first introduced in 1795, all metric units could be defined by reference to the standard metre or to the standard kilogram. In 1832 Carl Friedrich Gauss, when making the first absolute measurements of the Earth's magnetic field, needed standard units of time alongside the units of length and mass. He chose the second (rather than the minute or the hour) as his unit of time, thereby implicitly making the second a base unit of the metric system.[27] The hour and minute have however been "accepted for use within SI".[17] One second is now defined by taking the fixed numerical value of the caesium frequency ∆νCs, the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be 9192631770 when expressed in the unit Hz, which is equal to s−1.
During the 20th century it became apparent that the Earth's rotation was slowing down. This results in days becoming 1.4 milliseconds longer each century.[28] It was verified by comparing the calculated locations of eclipses of the Sun with those observed in antiquity going back to Chinese records of 763 BC[29] and Roman records of AD 484. The "sunrise" point of the eclipse on 14 January 484 was back-calculated and, using 20th century data, should have been close to Lisbon. Ancient records however record the "sunrise point" as being in the Ionian Sea, off the coast of Greece. This difference can be accounted for by assuming that the Earth is slowing down, and as a result a day in Roman times was a little over 0.02 seconds shorter than today.[30]
Until the advent of the atomic clock, the most reliable timekeeper available to mankind was the Earth's rotation. It was natural therefore that the astronomers under the auspices of the International Astronomical Union (IAU) took the lead in maintaining the standards relating to time.[31] In 1958, in anticipation of technology being able to measure the rate at which the Earth is slowing down, it was agreed that the second would be defined on the basis that in 1900 the Earth's average rotational speed gave an average day of exactly 60×60×24 = 86,400 seconds.[28] Astronomers from the US Naval Observatory (USNO) and the National Physical Laboratory determined a relationship between a specific microwave frequency emitted by an excited caesium-133 atom (about 9 GHz) and the back-calculated rate of rotation of the Earth in 1900. Their value was adopted in 1968 by the 13th CGPM as being the definition of the second.
Science and technology
During the 19th century, the British Association for the Advancement of Science took the lead in standardising units of measurement used in science and technology across the globe. Under the leadership of men like James Clerk Maxwell and Lord Kelvin, the metric system was the system of choice. Some units that they developed are still in use today; others have been superseded.
Scientists and engineers subsequently developed many other units of measure, some of which were discarded with the coming of SI. Scientific and technical units of measure frequently encountered by the layman today include:
- Volt (V) – the unit of electrical potential difference (often called voltage). Household electrical power supplies are usually rated at either 110–120 V (North America) or 220–240 V (Europe). An alkaline battery has a nominal voltage of 1.5 V.
- Pascal (Pa) – the unit of pressure: the excess air pressure in car tyres is typically around 200 kPa, equivalent to 200,000 Pa. The Earth's atmospheric pressure is about 100 kPa, and thus historically an alternative unit, the bar, was defined as 100 kPa. For weather forecasting, the hectopascal or millibar (both equivalent to 100 Pa) are widely used.
- Watt (W) – the unit of power. The watt is used in electrical, mechanical or any other contexts where power is measured. The imperial unit of power was the horsepower, which, ironically, was introduced by James Watt. Theoretically watts can be used anywhere that horsepower is used and vice versa. Watts and imperial horsepower are related by the relationship 1 HP = 746 W. (A "metric horsepower" has a different value.)
- Joule (J) – the unit of energy. Energy is defined as the product of power and time, the joule being defined as watts times seconds. In many countries energy quantities are often expressed in kilowatt-hours (kW⋅h). Since there are 1000 watts in a kilowatt and 3600 seconds in an hour, there are 1000 × 3600 joules in a kilowatt-hour; i.e. 1 kW⋅h = 3.6 MJ. (One megajoule is one million joules.)
- Hertz (Hz) – the unit of frequency: the number of times per second that some periodic phenomenon repeats itself; typically, the frequency of alternating current is either 50 Hz (Europe) or 60 Hz (North America), while an A tuning fork will vibrate at a frequency of about 440 Hz.
- Calorie (cal) – a non-SI unit of energy that is still used in the food industry (which uses calories and kilocalories interchangeably to denote the kilogram-calorie, or 1000 small calories). The calorie was defined as the energy required to raise the temperature of one gram of water by 1 °C. Any measurement that uses calories can also use joules, using the conversion 1 cal = 4.18 J, or 1 Cal = 1 kcal = 4.18 kJ for the dietary ("large") calorie.
Governance
The metric system of measure was first given a legal basis in 1795 by the French Revolution ary government. Article 5 of the law of 18 Germinal, Year III (7 April 1795) defined five units of measure.[4]
By 1870 the metric system had been adopted by most of the countries of Europe and on 20 May 1875 an international treaty known as the Convention du Mètre (Metre Convention) was signed by 17 to harmonise measurements between the states.[32][33] Initially the treaty only provided for the coordination of length and mass, but in 1921 the treaty was extended to cover all types of measurement. The treaty established the following organisations to conduct international activities relating to a uniform system for measurements:
- Conférence générale des poids et mesures (CGPM) (English: General Conference on Weights and Measures), an intergovernmental conference of official delegates of member nations and the supreme authority for all actions. The CGPM meets approximately every four years. Changes to the metric system are usually ratified at these meetings.
- Comité international des poids et mesures (CIPM) (English: International Committee for Weights and Measures), consisting of selected scientists and metrologists, which prepares and executes the decisions of the CGPM and is responsible for the supervision of the International Bureau of Weights and Measures. The CIPM meets every year.
- Bureau international des poids et mesures (BIPM) (English: International Bureau of Weights and Measures), a permanent laboratory and world centre of scientific metrology, the activities of which include the establishment of the basic standards and scales of the principal physical quantities, maintenance of the international prototype standards and oversight of regular comparisons between the international prototype and the various national standards.
In 1889 sets of new international prototype metres and kilograms made from a 90% platinum, 10% iridium alloy were manufactured by the London firm Johnson Matthey and delivered to the CGPM who calibrated them against the 1799 prototype. One master copy and a set of working copies were retained by the BIPM and the rest distributed to member nations. At intervals of about 25 years each nation returned their copies for re-calibration against the master copies.[34]
In 1921 the mandate for the CGPM and its subsidiary organisations was extended to include the standardisation of all physical measurements including electrical measurements, time and temperature.
References
- Alder, Ken (2002). The Measure of all Things – The Seven-Year Odyssey that Transformed the World. London: Abacus. ISBN 0-349-11507-9.
- ↑ 1.0 1.1 Alder, Ken (2002). The Measure of all Things – The Seven-Year Odyssey that Transformed the World. London: Abacus. pp. 2–3. ISBN 978-0-349-11507-8.
- ↑ 2.0 2.1 "History of measurement". Métrologie française. http://www.french-metrology.com/en/history/history-mesurement.asp. Retrieved 2011-02-06.
- ↑ Alder, Ken (2002). The Measure of all Things – The Seven-Year Odyssey that Transformed the World. London: Abacus. pp. 20–23. ISBN 978-0-349-11507-8.
- ↑ 4.0 4.1 4.2 "La loi du 18 Germinal an 3 (The law of 18 Germanial year 3) " la mesure [républicaine de superficie pour les terrains, égale à un carré de dix mètres de côté ""]. Le CIV (Centre d'Instruction de Vilgénis) – Forum des Anciens. http://aviatechno.free.fr/unites/nouveausys.php. Retrieved 2010-03-02.
- ↑ Alder, Ken (2002). "Prologue". The Measure of all Things – The Seven-Year Odyssey that Transformed the World. London: Abacus. ISBN 978-0-349-11507-8.
- ↑ "Historical context of the SI". U.S. National Institute of Standards and Technology. http://physics.nist.gov/cuu/Units/meter.html. Retrieved 14 February 2012.
- ↑ Thierry Thomasset. "Le stère" (in French). Université de Technologie de Compiègne. http://www.utc.fr/~tthomass/Themes/Unites/unites/infos/stere/Le%20stere.pdf. Retrieved 21 March 2011.
- ↑ International Bureau of Weights and Measures (2006), The International System of Units (SI) (8th ed.), pp. 138, 166, ISBN 92-822-2213-6, http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf
- ↑ Denis Février. "Un historique du mètre" (in French). Ministère de l'Economie, des Finances et de l'Industrie. http://www.industrie.gouv.fr/metro/aquoisert/metre.htm. Retrieved 2011-03-10.
- ↑ "The Metre Convention". International Bureau of Weights and Measures. http://www.bipm.org/en/convention/. Retrieved 2011-10-10.
- ↑ "The International System of Units (SI) – and the "New SI"…". International Bureau of Weights and Measures. http://www.bipm.org/en/si/. Retrieved 2011-10-10.
- ↑ Silvanus P. Thompson. "In the beginning...Lord Kelvin". International Electrotechnical Commission. http://www.iec.ch/about/history/beginning/lord_kelvin.htm. Retrieved 2011-05-10.
- ↑ Sabot, Thierry (1 October 2000). "Les poids et mesures sous l'Ancien Régime" (in French). histoire-genealogie. http://www.histoire-genealogie.com/spip.php?article396. Retrieved 2011-02-10.
- ↑ "ISMP's List of Error-Prone Abbreviations, Symbols and Dose Designations". Institute of Safe Medical Practices. 1 January 2004. http://www.ismp.org/tools/errorproneabbreviations.pdf. Retrieved 9 March 2012.
- ↑ "SI base units". International Bureau of Weights and Measures. http://www.bipm.org/en/si/base_units/. Retrieved 2011-10-10.
- ↑ "SI derived units". International Bureau of Weights and Measures. http://www.bipm.org/en/si/derived_units/. Retrieved 2011-10-10.
- ↑ 17.0 17.1 17.2 17.3 International Bureau of Weights and Measures (2006), The International System of Units (SI) (8th ed.), pp. 123,128, ISBN 92-822-2213-6, http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf
- ↑ This EU directive denotes "kilometres per hour" by "km/h" in all languages used:
"Directive 2000/7/EC of the European Parliament and of the Council of 20 March 2000 on speedometers for two- or three-wheel motor vehicles and amending Council Directive 92/61/EEC on the type-approval of two- or three-wheel motor vehicles". Commission of the European Community. http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32000L0007:en:NOT. Retrieved 25 October 2012. - ↑ meter. (2009). In Merriam-Webster Online Dictionary. Retrieved 8 December 2009.
- ↑ Alder, Ken (2002). The Measure of all Things – The Seven-Year Odyssey that Transformed the World. London: Abacus. ISBN 978-0-349-11507-8.
- ↑ Harmer, S.W.; Rezgui, N.; Bowring, N.; Luklinska, Z.; Ren, G.. "Determination of the Complex Permittivity of Textiles and Leather in the 14–40 GHz, mm wave band using a Free-W ave Transmittance Only Method". IET Microwaves, Antennas & Propagation: 8. https://uhra.herts.ac.uk/dspace/bitstream/2299/2418/1/902311.pdf. Retrieved 2011-10-08.
- ↑ "Bienvenue à Métrodiff !". http://www.metrodiff.org/cmsms/index.php?page=18_germinal_an_3.
- ↑ Halls, Steven B.; Hanson, John (2008). "Men's Weight Chart". http://www.halls.md/chart/men-weight-w.htm. Retrieved 2011-09-17.
- ↑ Halls, Steven B.; Hanson, John (2008). "Child Growth Charts of height weight and body mass index". http://www.halls.md/chart/child-growth/pediatric.htm. Retrieved 2011-09-17.
- ↑ "€ – our money – Common sides". European Central Bank. 2011. http://www.ecb.int/euro/coins/common/html/index.en.html. Retrieved 2011-09-17.
- ↑ Wang, Ming; Pittman, Roy (6 August 2008). "Resveratrol Content in Seeds of Peanut Germplasm Quantified by HPLC". Plant Genetic Resources: Characterization and Utilization. 7 (1): 80–83. doi:10.1017/s1479262108048247. http://www.ars.usda.gov/research/publications/publications.htm?seq_no_115=230823. Retrieved 12 October 2011.
- ↑ International Bureau of Weights and Measures (2006), The International System of Units (SI) (8th ed.), p. 109, ISBN 92-822-2213-6, http://www.bipm.org/utils/common/pdf/si_brochure_8_en.pdf
- ↑ 28.0 28.1 "Leap seconds". Time Service Department, U.S. Naval Observatory. Archived from the original on 2012-03-12. https://www.webcitation.org/666iH0vl1?url=http://tycho.usno.navy.mil/leapsec.html. Retrieved 2011-04-29.
- ↑ F. Richard Stephenson (1982). "Historical Eclipses". Scientific American 247 (4): 154–163. Bibcode: 1982SciAm.247d.154S. http://hbar.phys.msu.ru/gorm/atext/histecl.htm. Retrieved 2011-04-18.
- ↑ Christian, Eric (June 2000). "20. Speed of Earth's Rotation Slowing?". NASA.
- ↑ "The International System of Units". Satellite Today. 1 February 2000. http://www.satellitetoday.com/via/The-International-System-of-Units_32466_p3.html. Retrieved 2011-04-05.
- ↑ "The Metre Convention". International Bureau of Weights and Measures. http://www.bipm.org/en/convention/. Retrieved 5 December 2012.
- ↑ Text of the treaty: "Convention du mètre" (in French). http://www.bipm.org/utils/en/pdf/metre_convention.pdf. Retrieved 2011-03-08.
- ↑ Jabbour, Z.J; Yaniv, S.L (2001). "The Kilogram and Measurements of Mass and Force". J. Res. Natl. Inst. Stand. Technol. 106 (1): 25–46. doi:10.6028/jres.106.003. PMID 27500016. PMC 4865288. http://nvl.nist.gov/pub/nistpubs/jres/106/1/j61jab.pdf. Retrieved 2011-03-28.