Chemistry:Chemical traffic light experiment
The chemical traffic light is the reaction of the changing in color of the solution which is also related to the blue bottle experiment. One of the early formulas consists of glucose, sodium hydroxide, indigo carmine, and water. Another formula consists of indigo carmine, dye, ascorbic acid (Vitamin C), sodium bicarbonate, sodium chloride, copper(II) sulfate, sodium hydroxide and water.[1] By doing so, chemical waste and the level of corrosive chemicals is reduced. The amount of solid chemicals dissolved in the experiment could be reduced from 60 grams to 6 grams. And the pH could be lowered from 13 to 3 which is easier to neutralize the pH to 7 by adding baking soda before disposal.[2] Also, it is safer and the reactions also occur faster and are easier to perform.
At first, all chemicals are added together and the color appears yellow. After shaking, the color turns green and then changes to red after it is left untouched. When further observed, the color turns back to yellow, which is why the solution is called the chemical traffic light. This reaction can be repeated many times, but it needs additional oxygen or indigo carmine.
Chemical reaction
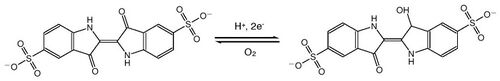
This experiment is all about oxidation and reduction of the solution where alkaline glucose solution is acting as a reducing agent. The glucose solution is added to the solution containing indicator (dye indigo carmine) the color changes occur. This reaction is also known as chemical clock experiment because concentrations of the products and reactants changed over the specific period.[3] When the solution is shaken, oxygen dissolves in the solution and oxidizes indigo carmine. Solution becomes red if a small amount of oxygen is dissolved, and green if all of indigo carmine is oxidized.[4] The solution will turn back to original yellow color when the concentration of oxygen level drops.[5][6]
Materials
- 1% indigo carmine indicator solution, 100 mL
- dextrose, 6 g
- Deionized water
- NaOH (Sodium Hydroxide), 0.5 M, 500 mL
- 1-L soda bottle, clear
Alternate methods
Preparing a bottle in advanced can be done as well, by make up all the solutions at the room temperature. Normally, it takes around 2 minutes to have full yellow color. However, after making the solution between 15–45 minutes, the color will be changed pretty much faster. After 45 minutes the color will be less impressive, which might be a good practical way to add more dye.[7]
Disposal
All liquids from the experiment can be safely poured down the sink and washed away with plenty of water.[7]
See also
References
- ↑ Rajchakit, Urawadee; Limpanuparb, Taweetham (2015-10-16). "Greening the Traffic Light: Air Oxidation of Vitamin C Catalyzed by Indicators" (in EN). Journal of Chemical Education. doi:10.1021/acs.jchemed.5b00630. Bibcode: 2016JChEd..93.1486R. http://pubs.acs.org/doi/abs/10.1021/acs.jchemed.5b00630.
- ↑ Wellman, Whitney E.; Noble, Mark E.; Healy, Tom (2003-05-01). "Greening the Blue Bottle" (in EN). Journal of Chemical Education 80 (5). doi:10.1021/ed080p537. Bibcode: 2003JChEd..80..537W. http://pubs.acs.org/doi/abs/10.1021/ed080p537.
- ↑ Communications, Frontiers (2015-10-22). "Chemistry Week: Chemical traffic light". https://blog.frontiersin.org/2015/10/22/chemistry-week-chemical-traffic-light/.
- ↑ "Chemical Traffic Light". http://practicum.melscience.com/experiments/chemical-traffic-light.html.
- ↑ http://chemistry.elmhurst.edu/demos/trafficlight2.htm
- ↑ Altott, April. "Traffic Light". https://blog.frontiersin.org/2015/10/22/chemistry-week-chemical-traffic-light/.
- ↑ 7.0 7.1 Fleming, Declan (6 May 2014). "Beyond the ‘blue bottle'". Education in Chemistry (Royal Society of Chemistry) 51 (3): 12–13. https://eic.rsc.org/exhibition-chemistry/beyond-the-blue-bottle/2000041.article. Retrieved 2016-12-03.
External links
- Beyond the blue bottle on Youtube