Chemistry:Thiazine
From HandWiki
Short description: Organic compound

| |
| Names | |
|---|---|
| Preferred IUPAC name
4H-1,4-Thiazine | |
| Other names
Parathiazine
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H5NS | |
| Molar mass | 99.15 g·mol−1 |
| Density | 0.8465 g/cm3 |
| Boiling point | 76.5 °C |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Thiazine /ˈθaɪəziːn/ is an organic compound containing a ring of four carbon, one nitrogen and one sulfur atom. There are three isomers of thiazine, 1,2-thiazine, 1,3-thiazine, and 1,4-thiazine, which differ by the arrangement of the nitrogen and sulfur atoms in the ring.
Derivatives of thiazine, often referred to as thiazines, are used for dyes, tranquilizers and insecticides.
Preparation
1,4-thiazine can be prepared from the corresponding dione using aluminium powder at high temperature.[1]
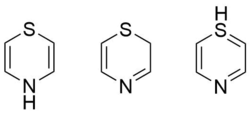
Tautomers
Three tautomers of 1,4-thiazine exist as above.
See also
- Methylene blue, contains a related ring with nitrogen and a positively charged sulfur atom
- Phenothiazine, a thiazine fused with two benzene rings
- Thiomorpholine, a saturated analog of thiazine
References
- ↑ Barkenbus, Charles; Landis, Phillip S. (February 1948). "The Preparation of 1,4-Thiazine". Journal of the American Chemical Society 70 (2): 684–685. doi:10.1021/ja01182a075. ISSN 0002-7863.
 |