Physics:Synchrotron radiation circular dichroism spectroscopy
Synchrotron radiation circular dichroism spectroscopy, commonly referred to as SRCD and also known as VUV-circular dichroism or VUVCD spectroscopy, is a powerful extension to the technique of circular dichroism (CD) spectroscopy, often used to study structural properties of biological molecules such as proteins and nucleic acids. The physical principles of SRCD are essentially identical to those of CD, in that the technique measures the difference in absorption (ΔA) of left (AL) and right (AR) circularly polarized light (ΔA=AL-AR) by a sample in solution. To obtain a CD(SRCD) spectrum the sample must be innately optically active (chiral), or, in some way be induced to have chiral properties, as only then will there be an observable difference in absorption of the left and right circularly polarized light. The major advantages of SRCD over CD arise from the ability to measure data over an extended wavelength range into the vacuum ultra violet (VUV) end of the spectrum. As these measurements are utilizing a light source with a higher photon flux (quantity of light stricking a given surface area) than a bench-top CD machine it means data are more accurate at these extended wavelengths because there is a larger signal over the background noise (the signal-to-noise ratio) and, generally, less sample is needed when recording the spectra and there is more information content available in the data.[1] Many beamlines now exist around the world to enable the measurement of SRCD data.
Origins
Extending the wavelength range for CD experiments had been both considered and instigated as far back as 1970. Three research groups had created their own "in-house" CD machines, with specialist lamps as their light source, to enable measurements in this range.[2] Synchrotron radiation (SR) had been proposed for use as the light source at a meeting in Brookhaven National Laboratory on Long Island in 1972,[2][3] however, it took a few years more before this came to fruition. Two research papers in 1980 reported the collection of CD data using SR as the light source for the experiments. Specifically, spectra were obtained in wavelength regions into the VUV range, from ~100 nanometers (nm) to ~200 nm, largely unavailable to laboratory-based bench-top spectrophotometers. Sutherland[4] et. al. focussed on the development of a versatile spectrophotometer capable of measuring CD, amongst other properties, in the VUV region of the spectrum,[5][6] while Snyder[7] and Rowe collected CD data from a small organic compound in the wavelength range 130.5 nm to 205 nm.[8]
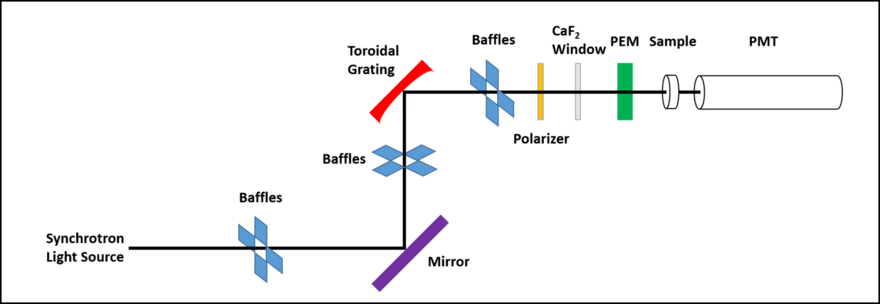
Simplified overview of an SRCD beamline setup
As shown in the diagram, a number of baffles are used throughout to remove possible stray light being reflected off the sides of the beamline tube. The use of only one mirror minimizes the loss of photon flux which is most important in the VUV region where reflectivity is poor relative to the visible wavelength range.[9]
The first constructed SRCD beamlines initially tried to utilize the intrinsic properties of the SR radiation produced, whereby there exists a "central" linearly-polarized component with, above and below this, equally opposing regions of circularly-polarized components. The premise for this was that the overall signal produced from a chiral sample would be enhanced by the absorption difference (the signal) derived from these circularly polarized features of the beam.[10] In an ideal situation this approach would work; however, this setup was modified such that all beamlines now include a linear polarizer (as shown) to remove these circularly polarized components. This was because even the minutest of movements in beam position (beam drift) led to unequal matching of the contributions of the circularly polarized components striking the sample and this, in turn, meant the SRCD signal produced was inaccurate and unreliable; often being irreproducible as a result.[10] Whereas cCD machines are purged throughout with nitrogen to minimize the absorption by oxygen of the light from the source xenon arc lamp, in an SRCD arrangement the beam passes through a calcium fluoride (CaF2), or similar "VUV-wavelengths transparent", window where everything before this point is in vacuum, and everything beyond is in nitrogen. The beam interacts with a photoelastic modulator (PEM) which consequently produces an alternating right- then left-circularly polarized beam and these now interact with the sample. The resultant absorption difference by the sample is measured and amplified by a photomultiplier tube (PMT) and from this the SRCD spectrum is recorded. The wavelength range that is utilized for SRCD studies is typically in the UV to VUV region and can go to below this; potentially from ~100 nm, up to the visible region, ~400 nm. The exact range over which data can be collected relies on the beamline set up, the sample preparation and the wavelength range of the PMT detector used. One of the primary factors limiting the lower wavelength cut off is the sample usually being in solution as a large water absorption band exists centred ~167 nm. This high absorption background swamps any possibility of measuring the very small CD difference signal, although use of deuterated water (D2O) as the solvent reduces the solvent absorption increasing the lower wavelength data collection range by ~10 nm.[11][12] Removing the solvating water completely, creating a film as a result, means that data can be recorded to significantly lower wavelengths, down to around ~130 nm.[13]
Advantages over conventional CD machines
The main advantages for SRCD over lab-based cCD machines arise from the use of the synchrotron light emission as the source. A number of biologically interesting absorption bands are found in the region between ~170 nm and ~350 nm. For proteins these come from their secondary and tertiary structures, while structural bands for nucleic acids, (DNA and RNA), and saccharides are also located in this region. However, for cCD machines the photon flux from the source reduces by around two orders of magnitude in the wavelength range from 250 nm down to 180 nm,[14] exactly in the region of most significance for these biological molecules. By contrast, typically, the photon flux for an SRCD beamline in this region is at least three orders of magnitude higher than a cCD machine, retaining that level down to ~150 nm.[14] The increased flux means the measured signals from the sample will be increased relative to the background noise, so there is a significant improvement in the signal-to-noise ratio of the sample. This will improve the accuracy of the data recorded meaning interpretation can be undertaken with more confidence in the results. A further advantage of the increased flux is that the concentration of the sample can be reduced while still retaining a significant increase in signal strength, so samples that are difficult to produce in quantity have more chance of producing usable CD data from SRCD rather than a cCD machine. Increasing the lower wavelength range provides more spectral data for analysis which means there is more information content[15] available in that data, meaning that more parameters, here secondary structure features in the protein structure, can be accurately determined.[15]
Technique growth and development
While the first reports of its use dated to 1980, it was a further two decades before the technique of SRCD took off largely due to the work of Bonnie Wallace at Birkbeck College, University of London. From around 2000, her aims in the field focused on both enhancing the collection of quality data through technical improvements, and on demonstrating "proof-of-principle" application studies, illustrating the novel information that SRCD offers. The construction on the Synchrotron Radiation Source (SRS) of the CD12 beamline[14] at Daresbury Laboratory, opened in 2005 under the auspices of the Centre for Protein and Membrane Structure and Dynamics (CPMSD)[1][16][17] of which Wallace was the Director, represented the first of the new, dedicated, second-generation SRCD beamlines. It was quickly identified that the high photon flux from CD12 was causing denaturation of the protein sample[18] but that this was resolvable by reducing the sample area being irradiated.[19] Later studies have identified the flux threshold limits that induce SRCD protein denaturation.[20] The input from the Wallace lab to the early years of SRCD development also included the introduction of calibration and standardization of SRCD and cCD spectrophotometers,[21][22] the creation of software to process the spectral data using CDtool,[23] and CDtoolX,[24] and to analyse the data using DichroWeb,[25][26][27][28] and the generation of reference data sets of proteins to support these data analyses.[29][30][31] Additionally, her lab produced sample cells with reduced pathlengths, and using material, (CaF2), transparent to VUV radiation which significantly enhanced the collection of data into the SRCD lower wavelength regions.[32]
New SRCD beamlines were constructed on various synchrotrons around the world. "ASTRID". https://www.isa.au.dk/facilities/astrid/astrid.asp. ring, in the Department of Physics and Astronomy of Aarhus University in Denmark , became a dedicated second-generation synchrotron in 2005. Ultimately this ring had two SRCD beamlines, UV1 and CD1, which migrated to the new third-generation ring, ASTRID2, in 2013/14, as AU-UV and AU-CD. SOLEIL synchrotron, near Paris, France , commissioned a dedicated SRCD beamline, DISCO, around 2005.[33] At Hiroshima Synchrotron Radiation Center, also known as HiSOR, a VUVCD beamline was constructed over the same period, while a little later in 2009, an SRCD beamline was commissioned in Beijing, China . This particular beamline is unique in that the synchrotron which acts as its light source is also the electron carrying ring of the Beijing Electron Positron Collider.[34] The SRS closed in 2008[35] being superseded in the UK by the Diamond Light Source on which an SRCD beamline opened for use in 2010.[36] With the SRS closure the CD12 SRCD beamline was moved to, and installed on, the ANKA Synchrotron Radiation Facility, (now called KARA), part of Karlsruhe Institute of Technology (KIT), in Karlsruhe, Germany . This beamline opened for users in 2011[13] but was closed in 2021. Currently under construction (as of June 2023) on the Sirius synchrotron light source in Campinas, Brazil , is a new SRCD beamline, CEDRO.[37]
Examples of applications
Highlighting a few of the published works that have employed SRCD in their research studies best illustrates the power of this technique.
Improved conformational analysis due to increased signal-to-noise ratio
Cataracts are the primary cause of blindness in humans and mutations in one particular protein, γD-crystallin, have been linked to a number of congenital forms of this disease.[38] An amino acid mutation, proline (P) to threonine (T) at position 23 of the polypeptide chain has been linked to at least four different forms of this ailment. SRCD investigations were conducted on the wild-type protein and two variants, the P23T mutant found in the disease, and a related modification, P23S (proline to serine, a chemically similar amino acid to threonine), to establish the nature of the cause of cataract formation.[39] Two possible reasons were suggested as the causative factor; the reduced solubility of the mutant protein, or an instability in the structure of the protein being introduced by the mutation. Significantly, because the mutant had limited solubility, lab-based CD machines were only able to provide very noisy spectra and the data were uninterpretable as a result. However, the SRCD spectra produced had very low noise associated with their data, including the mutant, and showed clearly that the structures of the wild-type, the mutant, and the related protein all had very similar conformations. These data also established that the mutant retained stability to thermal denaturation, very similar to that of the wild-type protein. The data confirmed that the causative factor for the cataracts was the reduction in solubility associated with the P23T mutation and not changes in the stability of the protein.[39]
Because of a high degree of flexibility, it had proven difficult to determine the structure of the extramembranous C-terminal domain of bacterial voltage-gated sodium channels. Using a series of synthesised channels where this C-terminal domain had been truncated, in some cases by a single amino acid difference between the constructs, the Wallace lab used SRCD to successfully identify the structure of this region.[40]
Intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs)
Intrinsically disordered proteins (IDPs) have very limited innate structure in solution but gain shape specifically when interacting with partner molecules such as proteins or RNA; however, their resultant structure is often dictated by this interaction. In addition, some proteins have sections of sequence without structure, termed intrinsically disordered regions (IDRs), that also gain structure on interaction. Having different shapes with different partners means they are functionally, as well as structurally flexible, making them centrally important to signalling pathways[41] and as regulation/control factors[42] for example. IDPs (and IDRs if capable of being isolated from the rest of the protein) have a distinct SRCD spectral appearance in solution which means that changes in their spectra that arise through interactions offer an ideal opportunity to gain insight into what is happening both structurally and functionally. In addition, SRCD studies have demonstrated that when the solvating water is removed from these proteins, generating a film, there is a gain in structure and more CD transition bands can be measured into the lower VUV wavelength region because the water absorption band is not present[43]
Myelin is the insulating sheath that is formed in the central (CNS) and peripheral nervous systems (PNS) to surround nerve cell axons thereby increasing and maintaining the electrical impulse, the action potential, sent along them. Formed mostly of lipids, there are specific proteins within the myelin components whose roles are to structure the myelin into linked layers. Two of these proteins are myelin basic protein (MBP), an IDP primarily in the CNS, and myelin protein zero (P0) which contains an IDR section (P0ct) and is key within the PNS. MBP and P0ct were employed in a study[44] which used SRCD data as a key factor to establish if there was any significance to the predictions of their IDP and IDR protein structures generated by Alphafold2, an artificial intelligence program developed by DeepMind. PDB2CD,[45] a package that generates SRCD spectra from protein atomic coordinates, was used to calculate spectra from the Alphafold2 structures, and these spectra were then compared against SRCD experimental spectra collected from the MBP and P0ct proteins in various ambient conditions; solution, detergent and lipid-bound states. The study reported that from the SRCD comparisons, the structures predicted by Alphafold2 for MBP and P0ct bore a strong resemblance to those when they were bound to the lipid membrane.[44]
Sugar modification of protein SRCD signals
One major feature found in protein structures is the addition of sugars (glycosylation) to specific amino acid residues by post translational modification. Complex sugar structures can be connected to these sites, and this can substantially modify the properties of these proteins, a main reason for their presence. Attached sugars can assist in folding some proteins to their correct shape; so, affecting a proteins’ structure is a possible outcome. SRCD is ideally well suited to determining any conformational differences that might arise from different ambient environments directly because of the extended wavelength range into the VUV region which provides greater information content. However, attached sugars can contribute to the SRCD signal because their transitions are located more towards the VUV end of the spectrum. This means that their presence can cause a problem in obtaining an accurate measure of the secondary structure content of the protein as a result. Matsuo.[46] and Gekko produced the landmark study of VUVCD spectra of selected saccharides, thereby demonstrating that glycoproteins would have a contribution to their spectra from their sugar content.[47] From this and further studies[48] they demonstrated that the SRCD spectral characteristics that arose from sugars could be attributed to many factors within their conformations: the configuration of the hydroxyl group about the C1 atom of the saccharide (alpha or beta conformation, or almost axial or equatorial to the plane of the sugar ring respectively), the axial or equatorial positioning of the remaining hydroxyl groups, the trans or gauche nature of the C5 hydroxymethyl group, and the glycosidic linkage (either 1-4 or 1-6) between sugar monomers. Utilising this information, the Wallace group investigated the glycosylation of the voltage-gated sodium channel in experiments that relied on the fact that a CD(SRCD) spectrum of a mixture of components is the sum of all those components present.[49] The aim was to establish if there were differences in the three-dimensional structure of the channel with and without sugars attached to the structure; did glycosylation play any significant role in the function of these channels when sugars were attached? Three experimental sets of SRCD spectra were collected; the non-glycosylated and glycosylated channel structures and a further one of the isolated sugar components that combined to form those attached to the channel. Taking away the spectrum of the non-glycosylated channel from that of the glycosylated they demonstrated that the resultant difference spectrum corresponded to that of the sugar components. This meant that there were no structural differences between the glycosylated and non-glycosylated channel structures, so sugar attachment played no key role in their function[49]
Conformational changes of globular proteins at the oil-water interface
First studied in 2010 via this method,[50] a recent investigation[51] used SRCD to examine the differences in structure in solution and when at the oil-water interface, of peptides derived from seaweed, bacteria and potatoes as potential emulsifying agents. Of these studied, the peptide from bacteria proved to be the most effective at being both an emulsifying agent and stabilising antioxidant compound.[51]
Existing beamlines
A number of SRCD beamlines exist, or are being constructed ((As of 2023)), around the world as listed in the table.
| Facility Name(Funders) | Location | Country | Ring Energy | Beamline Name |
|---|---|---|---|---|
| ASTRID2[52] | Aarhus University | Denmark | 580 MeV | AU-UV |
| ASTRID2 | Aarhus University | Denmark | 580 MeV | AU-CD |
| SOLEIL[53][54] | Near Paris | France | 2.75 GeV | DISCO[a] |
| HiSOR[55] | Hiroshima University | Japan | 150 MeV | BL-12 |
| Beijing Synchrotron Radiation Facility[56][b] | Beijing | China | 2.5 GeV | 4B8[57] |
| DIAMOND[58][59] | Near Oxford[60] | United Kingdom | 3 GeV | B23[c] |
| Sirius[61][62] | Campinas | Brazil | 3 GeV | CEDRO[d] |
a As of 2022 components from former SRCD beamline CD12[13] (on KARA) are now installed on the DISCO beamline
b This facility also runs as part of the Beijing Electron Positron Collider (BEPC)[34]
c Two modules (A and B) exist on this beamline
d This beamline is under construction and received its "first light" as of June 2023[63]
References
- ↑ 1.0 1.1 Wallace, B. A. (2000). "Synchrotron radiation circular-dichroism spectroscopy as a tool for investigating protein structures". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 7 (5): 289–295. doi:10.1107/s0909049500009262. ISSN 0909-0495. PMID 16609210.
- ↑ 2.0 2.1 Pysh, E. S. (1976). "Optical activity in the vacuum ultraviolet". Annual Review of Biophysics and Bioengineering (Annual Reviews) 5 (1): 63–75. doi:10.1146/annurev.bb.05.060176.000431. ISSN 0084-6589. PMID 782346.
- ↑ "Research Applications of Synchrotron Radiation Report 1972". https://digital.library.unt.edu/ark:/67531/metadc1023073/m2/1/high_res_d/4340726.pdf.
- ↑ "College of Science and Mathematics". https://www.augusta.edu/scimath/index.php.
- ↑ Sutherland, J. C.; Desmond, E. J.; Takacs, P. Z. (1980). "Versatile spectrometer for experiments using synchrotron radiation at wave-lengths greater than 100 nm". Nuclear Instruments and Methods (Elsevier BV) 172 (1–2): 195–199. doi:10.1016/0029-554x(80)90634-5. ISSN 0029-554X. Bibcode: 1980NucIM.172..195C. https://digital.library.unt.edu/ark:/67531/metadc1203790/.
- ↑ Sutherland, J. C.; Desmond, E. J.; Takacs, P. Z. (January 1, 1979). "Versatile spectrometer for experiments using synchrotron radiation at wavelengths greater than 100 nm". https://www.osti.gov/biblio/6386631.
- ↑ "Dr. Patricia Ann Snyder - Department of Chemistry and Biochemistry : Florida Atlantic University - Charles E. Schmidt College of Science". https://chemistry.fau.edu/directory/snyder.php.
- ↑ Snyder, P. A.; Rowe, E. M. (1980). "The first use of synchrotron radiation for vacuum ultraviolet circular dichroism measurements". Nuclear Instruments and Methods (Elsevier BV) 172 (1–2): 345–349. doi:10.1016/0029-554x(80)90657-6. ISSN 0029-554X. Bibcode: 1980NucIM.172..345S.
- ↑ Wallace, Bonnie Ann, ed (2009). "Measurement of circular dichroism and related spectroscopies with conventional and synchrotron light sources: Theory and instrumentation". Modern techniques for circular dichroism and synchrotron radiation circular dichroism spectroscopy. Amsterdam: IOS Press. p. 19-72. ISBN 978-1-4416-1665-4. OCLC 427189579.
- ↑ 10.0 10.1 Wallace, Bonnie Ann, ed (2009). "Calibration techniques for circular dichroism and synchrotron radiation circular dichroism spectroscopy". Modern techniques for circular dichroism and synchrotron radiation circular dichroism spectroscopy. Amsterdam: IOS Press. p. 73-90. ISBN 978-1-4416-1665-4. OCLC 427189579.
- ↑ Stipanovic, A. J.; Stevens, E. S.; Gekko, K. (1980). "Vacuum ultraviolet circular dichroism of dextran". Macromolecules (American Chemical Society (ACS)) 13 (6): 1471–1473. doi:10.1021/ma60078a021. ISSN 0024-9297. Bibcode: 1980MaMol..13.1471S.
- ↑ Sutherland, J. C.; Lin, B.; Mugavero, J. A.; Trunk, J.; Tomasz, M.; Vacuum ultraviolet circular dichroism of dextran, R.; Marky, L.; Breslauer, K. J. (1986). "Vacuum ultraviolet circular dichroism of double stranded nucleic acids". Photochemistry and Photobiology (Wiley) 44 (3): 295–301. doi:10.1111/j.1751-1097.1986.tb04667.x. ISSN 0031-8655. PMID 3786449.
- ↑ 13.0 13.1 13.2 Bürck, J.; Roth, S.; Windisch, D.; Wadhwani, P.; Moss, D.; Ulrich, A. S. (2015). "UV-CD12: synchrotron radiation circular dichroism beamline at ANKA". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 22 (3): 844–852. doi:10.1107/s1600577515004476. ISSN 1600-5775. PMID 25931105.
- ↑ 14.0 14.1 14.2 Clarke, D. T.; Jones, G. (2004). "CD12: a new high-flux beamline for ultraviolet and vacuum-ultraviolet circular dichroism on the SRS, Daresbury". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 11 (2): 142–149. doi:10.1107/s0909049503024142. ISSN 0909-0495. PMID 14960778.
- ↑ 15.0 15.1 Hennessey, J. P.; Johnson Jr, W. C. (1981). "Information content in the circular dichroism of proteins". Biochemistry (American Chemical Society (ACS)) 20 (5): 1085–1094. doi:10.1021/bi00508a007. ISSN 0006-2960. PMID 7225319.
- ↑ "House of Commons - Science and Technology - Written Evidence". https://publications.parliament.uk/pa/cm200304/cmselect/cmsctech/6/6we04.htm.
- ↑ Clarke, D. T.; Bowler, M. A.; Fell, B. D.; Flaherty, J. V.; Grant, A. F.; Jones, G. R.; Martin-fernandez, M. L.; Shaw, D. A. et al. (2000). "A high aperture beamline for vacuum ultraviolet circular dichroism on the srs". Synchrotron Radiation News (Informa UK Limited) 13 (2): 21–27. doi:10.1080/08940880008261066. ISSN 0894-0886. Bibcode: 2000SRNew..13...21C.
- ↑ Wien, F.; Miles, A. J.; Lees, J. G.; Hoffmann, S. V.; Wallace, B. A. (2005). "VUV irradiation effects on proteins in high-flux synchrotron radiation circular dichroism spectroscopy". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 12 (4): 517–523. doi:10.1107/s0909049505006953. ISSN 0909-0495. PMID 15968132.
- ↑ Janes, R. W.; Cuff, A. L. (2005). "Overcoming protein denaturation caused by irradiation in a high-flux synchrotron radiation circular dichroism beamline". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 12 (4): 524–529. doi:10.1107/s0909049505007703. ISSN 0909-0495. PMID 15968133.
- ↑ Miles, A. J.; Janes, R. W.; Brown, A.; Clarke, D. T.; Sutherland, J. C.; Tao, Y.; Wallace, B. A.; Hoffmann, S. V. (2008). "Light flux density threshold at which protein denaturation is induced by synchrotron radiation circular dichroism beamlines". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 15 (4): 420–422. doi:10.1107/s0909049508009606. ISSN 0909-0495. PMID 18552437.
- ↑ Miles, A. J.; Wien, F.; Lees, J. G.; Rodger, A.; Janes, R. W.; Wallace, B. A. (2003). "Calibration and standardisation of synchrotron radiation circular dichroism and conventional circular dichroism spectrophotometers". Spectroscopy (Hindawi Limited) 17 (4): 653–661. doi:10.1155/2003/379137. ISSN 0712-4813.
- ↑ Miles, A. J.; Wien, F.; Lees, J. G.; Wallace, B. A. (2005). "Calibration and standardisation of synchrotron radiation and conventional circular dichroism spectrometers. Part 2: Factors affecting magnitude and wavelength". Spectroscopy (Hindawi Limited) 19 (1): 43–51. doi:10.1155/2005/263649. ISSN 0712-4813.
- ↑ Lees, J. G.; Smith, B. R.; Wien, F.; Miles, A. J.; Wallace, B. A. (2004). "CDtool—an integrated software package for circular dichroism spectroscopic data processing, analysis, and archiving". Analytical Biochemistry (Elsevier BV) 332 (2): 285–289. doi:10.1016/j.ab.2004.06.002. ISSN 0003-2697. PMID 15325297.
- ↑ Miles, A. J.; Wallace, B. A. (2018). "CDtoolX, a downloadable software package for processing and analyses of circular dichroism spectroscopic data". Protein Science (Wiley) 27 (9): 1717–1722. doi:10.1002/pro.3474. ISSN 0961-8368. PMID 30168221.
- ↑ Lobley, A.; Whitmore, L.; Wallace, B. A. (2002). "DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra". Bioinformatics (Oxford University Press (OUP)) 18 (1): 211–212. doi:10.1093/bioinformatics/18.1.211. ISSN 1367-4803. PMID 11836237.
- ↑ Whitmore, L.; Wallace, B. A. (2004). "DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data". Nucleic Acids Research (Oxford University Press (OUP)) 32 (Web Server): W668–W673. doi:10.1093/nar/gkh371. ISSN 0305-1048. PMID 15215473.
- ↑ Whitmore, L.; Wallace, B. A. (2008). "Protein secondary structure analyses from circular dichroism spectroscopy: Methods and reference databases". Biopolymers (Wiley) 89 (5): 392–400. doi:10.1002/bip.20853. ISSN 0006-3525. PMID 17896349.
- ↑ Miles, A. J.; Ramalli, S. G.; Wallace, B. A. (2021). "DichroWeb, a website for calculating protein secondary structure from circular dichroism spectroscopic data". Protein Science (Wiley) 31 (1): 37–46. doi:10.1002/pro.4153. ISSN 0961-8368. PMID 34216059.
- ↑ Lees, J. G.; Miles, A. J.; Wien, F.; Wallace, B. A. (2006). "A reference database for circular dichroism spectroscopy covering fold and secondary structure space". Bioinformatics (Oxford University Press (OUP)) 22 (16): 1955–1962. doi:10.1093/bioinformatics/btl327. ISSN 1460-2059. PMID 16787970.
- ↑ Abdul-Gader, A.; Miles, A. J.; Wallace, B. A. (2011). "A reference dataset for the analyses of membrane protein secondary structures and transmembrane residues using circular dichroism spectroscopy". Bioinformatics (Oxford University Press (OUP)) 27 (12): 1630–1636. doi:10.1093/bioinformatics/btr234. ISSN 1460-2059. PMID 21505036.
- ↑ Evans, P.; Bateman, O.A.; Slingsby, C.; Wallace, B. A. (2007). "A reference dataset for circular dichroism spectroscopy tailored for the βγ-crystallin lens proteins". Experimental Eye Research (Elsevier BV) 84 (5): 1001–1008. doi:10.1016/j.exer.2007.01.016. ISSN 0014-4835. PMID 17400211.
- ↑ Wien, F.; Wallace, B. A. (2005). "Calcium fluoride micro cells for synchrotron radiation circular dichroism spectroscopy". Applied Spectroscopy (SAGE PubliCalcium fluoride micro cells for synchrotron radiation circular dichroism spectroscopycations) 59 (9): 1109–1113. doi:10.1366/0003702055012546. ISSN 0003-7028. PMID 16197633. Bibcode: 2005ApSpe..59.1109W.
- ↑ Miron, S.; Réfregiers, M.; Gilles, A-M.; Maurizot, J-C. (2005). "New synchrotron radiation circular dichroism end-station on DISCO beamline at SOLEIL synchrotron for biomolecular analysis". Biochimica et Biophysica Acta (BBA) - General Subjects (Elsevier BV) 1724 (3): 425–431. doi:10.1016/j.bbagen.2005.05.012. ISSN 0304-4165. PMID 16026934.
- ↑ 34.0 34.1 "Chinese Academy of Sciences". https://lssf.cas.cn/en/facilities-view.jsp?id=ff8080814ff56599014ff5a8f2680059.
- ↑ List of synchrotron radiation facilities
- ↑ Siligardi, G.; Hussain, R.; Myatt, D.; Jávorfi, T. (2010). "B23 circular dichroism beamline at the Diamond Light Source". Diamond Light Source Proceedings (Cambridge University Press (CUP)) 1 (SRMS-7). doi:10.1017/s2044820110000092. ISSN 2044-8201.
- ↑ "CEDRO BEAMLINE". https://lnls.cnpem.br/facilities/cedro-en/.
- ↑ Pande, A.; Pande, J.; Asherie, N.; Lomakin, A.; Ogun, O.; King, J.; Benedek, G. B. (2001). "Crystal cataracts: Human genetic cataract caused by protein crystallization". Proceedings of the National Academy of Sciences 98 (11): 6116–6120. doi:10.1073/pnas.101124798. ISSN 0027-8424. PMID 11371638. Bibcode: 2001PNAS...98.6116P.
- ↑ 39.0 39.1 Evans, P.; Wyatt, K.; Wistow, G. J.; Bateman, O. A.; Wallace, B. A.; Slingsby, C. (2004). "The P23T Cataract mutation causes loss of solubility of folded γD-crystallin". Journal of Molecular Biology (Elsevier BV) 343 (2): 435–444. doi:10.1016/j.jmb.2004.08.050. ISSN 0022-2836. PMID 15451671.
- ↑ Powl, A. M.; O’Reilly, A. O.; Miles, A. J.; Wallace, B. A. (2010). "Synchrotron radiation circular dichroism spectroscopy-defined structure of the C-terminal domain of NaChBac and its role in channel assembly". Proceedings of the National Academy of Sciences 107 (32): 14064–14069. doi:10.1073/pnas.1001793107. ISSN 0027-8424. PMID 20663949. Bibcode: 2010PNAS..10714064P.
- ↑ Cornish, J.; Chamberlain, S. G.; Owen, D.; Mott, H. R. (2020). "Intrinsically disordered proteins and membranes: a marriage of convenience for cell signalling?". Biochemical Society Transactions (Portland Press Ltd.) 48 (6): 2669–2689. doi:10.1042/bst20200467. ISSN 0300-5127. PMID 33155649.
- ↑ Babu, M. M.; van der Lee, R.; de Groot, N. S.; Gsponer, J. (2011). "Intrinsically disordered proteins: regulation and disease". Current Opinion in Structural Biology (Elsevier BV) 21 (3): 432–440. doi:10.1016/j.sbi.2011.03.011. ISSN 0959-440X. PMID 21514144.
- ↑ Yoneda, J. S.; Miles, A. J.; Araujo, A. P. U.; Wallace, B. A. (2017). "Differential dehydration effects on globular proteins and intrinsically disordered proteins during film formation". Protein Science (Wiley) 26 (4): 718–726. doi:10.1002/pro.3118. ISSN 0961-8368. PMID 28097742.
- ↑ 44.0 44.1 Krokengen, O. C.; Raasakka, A.; Kursula, P. (2023). "The intrinsically disordered protein glue of the myelin major dense line: Linking AlphaFold2 predictions to experimental data". Biochemistry and Biophysics Reports (Elsevier BV) 34: 101474. doi:10.1016/j.bbrep.2023.101474. ISSN 2405-5808. PMID 37153862.
- ↑ Mavridis, L.; Janes, R. W. (2016-09-20). "PDB2CD: a web-based application for the generation of circular dichroism spectra from protein atomic coordinates". Bioinformatics (Oxford University Press (OUP)) 33 (1): 56–63. doi:10.1093/bioinformatics/btw554. ISSN 1367-4803. PMID 27651482.
- ↑ "Koichi Matsuo, Hiroshima Synchrotron Radiation Center". https://seeds.office.hiroshima-u.ac.jp/profile/en.76158b13652f571b520e17560c007669.html.
- ↑ Matsuo, K.; Gekko, K. (2004). "Vacuum-ultraviolet circular dichroism study of saccharides by synchrotron radiation spectrophotometry". Carbohydrate Research (Elsevier BV) 339 (3): 591–597. doi:10.1016/j.carres.2003.11.019. ISSN 0008-6215. PMID 15013395.
- ↑ Gekko, K.; Yonehara, R.; Sakurada, Y.; Matsuo, K. (2005). "Structure analyses of biomolecules using a synchrotron radiation circular dichroism spectrophotometer". Journal of Electron Spectroscopy and Related Phenomena (Elsevier BV) 144-147: 295–297. doi:10.1016/j.elspec.2005.01.106. ISSN 0368-2048.
- ↑ 49.0 49.1 Cronin, N. B.; O'Reilly, A.; Duclohier, H.; Wallace, B. A. (2004). "Effects of Deglycosylation of Sodium Channels on Their Structure and Function". Biochemistry (American Chemical Society (ACS)) 44 (2): 441–449. doi:10.1021/bi048741q. ISSN 0006-2960. PMID 15641768.
- ↑ Zhai, J.; Miles, A. J.; Pattenden, L. K.; Lee, T-H.; Augustin, M. A.; Wallace, B. A.; Aguilar, M-I.; Wooster, T. J. (2010). "Changes in β-Lactoglobulin conformation at the oil/water interface of emulsions studied by synchrotron radiation circular dichroism spectroscopy". Biomacromolecules (American Chemical Society (ACS)) 11 (8): 2136–2142. doi:10.1021/bm100510j. ISSN 1525-7797. PMID 20690721.
- ↑ 51.0 51.1 Yesiltas, B.; Soria Caindec, A. M.; García-Moreno, P. J.; Echers, S. G.; Olsen, T. H.; Jones, N. C.; Hoffmann, S. V.; Marcatili, P. et al. (2023). "Physical and oxidative stability of fish oil-in-water emulsions stabilized with emulsifier peptides derived from seaweed, methanotrophic bacteria and potato proteins". Colloids and Surfaces A: Physicochemical and Engineering Aspects (Elsevier BV) 663: 131069. doi:10.1016/j.colsurfa.2023.131069. ISSN 0927-7757.
- ↑ "ISA - Centre for Storage Ring Facilities, Aarhus". https://www.isa.au.dk/.
- ↑ CNRS
- ↑ CEA
- ↑ "Hiroshima Synchrotron Radiation Center, Hiroshima University". http://www.hsrc.hiroshima-u.ac.jp/english/index.html.
- ↑ Institute of High Energy Physics, Chinese Academy of Sciences
- ↑ Tao, Y.; Huang, Y.; Gao, Z.; Zhuang, H.; Zhou, A.; Tan, Y.; Li, D.; Sun, S. (2009). "Developing VUV spectroscopy for protein folding and material luminescence on beamline 4B8 at the Beijing Synchrotron Radiation Facility". Journal of Synchrotron Radiation (International Union of Crystallography (IUCr)) 16 (6): 857–863. doi:10.1107/s0909049509037236. ISSN 0909-0495. PMID 19844024.
- ↑ UK Research & Innovation (UKRI)
- ↑ Wellcome Trust
- ↑ "Harwell Science and Innovation Campus". https://www.harwellcampus.com/.
- ↑ Ministry of Science, Technology, Innovation and Communication (Brazil)
- ↑ São Paulo Research Foundation
- ↑ "First Light". https://lnls.cnpem.br/sirius-updates/cedro-research-station-receives-its-first-ultraviolet-beamline-generated-by-sirius/. |
 |