Biology:Myelin protein zero
Myelin protein zero (P0, MPZ) is a single membrane glycoprotein[1] which in humans is encoded by the MPZ gene. P0 is a major structural component of the myelin sheath in the peripheral nervous system (PNS).[2] Myelin protein zero is expressed by Schwann cells and accounts for over 50% of all proteins in the peripheral nervous system, making it the most common protein expressed in the PNS.[2] Mutations in myelin protein zero can cause myelin deficiency and are associated with neuropathies like Charcot–Marie–Tooth disease and Dejerine–Sottas disease.[3]
Structure
| Myelin-PO_C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
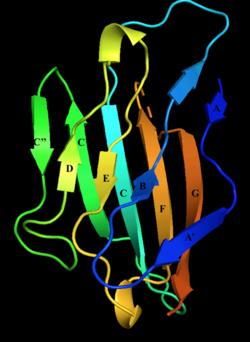 Structure of myelin protein zero's extracellular domain with labelled beta strands. Strands D, E, B, and A make up one beta sheet, Strands A', G, F, C, C', C'' make up the other beta sheet. | |||||||||
| Identifiers | |||||||||
| Symbol | Myelin-PO_C | ||||||||
| Pfam | PF10570 | ||||||||
| InterPro | IPR019566 | ||||||||
| OPM superfamily | 193 | ||||||||
| OPM protein | 3oai | ||||||||
| Membranome | 213 | ||||||||
| |||||||||
In humans, the gene that encodes myelin protein zero is located on chromosome 1 near the Duffy locus or the Duffy antigen/chemokine receptor. The gene is about 7,000 bases long and is divided into 6 exons. In total, myelin protein zero is 219 amino acids long[2] and has many basic amino acid residues.[4]
Myelin protein zero consists of an extracellular N-terminal domain (amino acids 1–124), a single transmembrane region (125–150), and a smaller positively charged intracellular region (151–219).[2][5][6] Its cytoplasmic domain is highly positively charged but presumably does not fold into a globular structure.[7] The extracellular domain is structurally similar to the immunoglobulin domain[4] and therefore the protein is considered as belonging to immunoglobulin superfamily.[8]
Besides existing as a monomer, myelin protein zero is also known to form dimers and tetramers with other myelin protein zero molecules in vertebrates.[9]
Function
The myelin sheath is a multi-layered membrane, unique to the nervous system, that functions as an insulator to greatly increase the velocity of axonal impulse conduction. Myelin protein zero, absent in the central nervous system,[10] is a major component of the myelin sheath in peripheral nerves. Mutations that disrupt the function of myelin protein zero can lead to less expression of myelin and degeneration of myelin sheath in the peripheral nervous system.[11] Currently, myelin protein zero expression is postulated to be produced by signals from the axon. However, more details about the regulation of myelin protein zero are unknown.[2]
It is postulated that myelin protein zero is a structural element in the formation and stabilization of peripheral nerve myelin.[5] Myelin protein zero is also hypothesized to serve as a cell adhesion molecule, holding multiple layers of myelin together.[6] When a myelinating cell wraps its membrane around an axon multiple times, generating multiple layers of myelin, myelin protein zero helps keep these sheets compact by serving as a "glue" that keeps the layers of myelin together.[7] It does so by holding its characteristic coil structure together by the electrostatic interactions[4] of its positively charged intracellular domain with acidic lipids in the cytoplasmic face of the opposite bilayer.[10] and by interaction between hydrophobic globular 'heads' of adjacent extracellular domains.[10]
Myelin protein zero's function is similar to the function of other proteins with immunoglobin domains like polyimmunoglobin and T4 protein. These proteins function as binding and adhesion molecules and participate in homotypic interactions, or interactions that involve two similar proteins.[5] Myelin protein zero holds together the myelin sheath by participating in homotypic interactions with other myelin protein zero proteins. Myelin protein zero's extracellular domain binds to the myelin sphingolipid membrane and holds together myelin layers using homotypic interactions with other myelin protein zero extracellular domains,[3] and with extracellular tryptophan residues interacting with the membrane.[4]
Myelin protein zero has also been demonstrated to interact with other proteins like peripheral myelin protein 22.[12] However, at this point the purpose of these interactions has not yet been determined.[12]
Associations with neuropathy
Mutations in myelin protein zero are known to cause myelin degeneration and neuropathy.[3] Mutations that reduce myelin protein zero's adhesion function or its ability to participate in homeotypic interactions with other myelin protein zero proteins are thought to cause neuropathy.[13] Mutations to myelin protein zero can lead to issues with the development of myelin early on in life or myelin degeneration on the axon later on in life.[8] Some mutations can cause neuropathy in infancy like Derjerine-Sottas disease while other mutations can cause neuropathy within the first two decades of life like Charcot-Marie-Tooth disease.[3] Adding a charged amino acid or changing a cysteine residue in the extracellular membrane can lead to neuropathy onset early on. Truncating the cytoplasmic domain or changing the tertiary structure of myelin protein zero can also result in neuropathy[3] because the cytoplasmic domain has been demonstrated to be necessary for homotypic interactions.[8]
References
- ↑ "Neuroactive steroids and peripheral myelin proteins". Brain Research. Brain Research Reviews 37 (1–3): 360–71. November 2001. doi:10.1016/s0165-0173(01)00140-0. PMID 11744100.
- ↑ Jump up to: 2.0 2.1 2.2 2.3 2.4 "Peripheral neuropathies caused by mutations in the myelin protein zero". Journal of the Neurological Sciences 242 (1–2): 55–66. March 2006. doi:10.1016/j.jns.2005.11.015. PMID 16414078.
- ↑ Jump up to: 3.0 3.1 3.2 3.3 3.4 "Phenotypic clustering in MPZ mutations". Brain 127 (Pt 2): 371–84. February 2004. doi:10.1093/brain/awh048. PMID 14711881.
- ↑ Jump up to: 4.0 4.1 4.2 4.3 "Crystal structure of the extracellular domain from P0, the major structural protein of peripheral nerve myelin". Neuron 17 (3): 435–49. September 1996. doi:10.1016/s0896-6273(00)80176-2. PMID 8816707.
- ↑ Jump up to: 5.0 5.1 5.2 "Isolation and sequence of a cDNA encoding the major structural protein of peripheral myelin". Cell 40 (3): 501–8. March 1985. doi:10.1016/0092-8674(85)90198-9. PMID 2578885.
- ↑ Jump up to: 6.0 6.1 "Isolation and analysis of the gene encoding peripheral myelin protein zero". Neuron 1 (1): 73–83. March 1988. doi:10.1016/0896-6273(88)90211-5. PMID 2483091.
- ↑ Jump up to: 7.0 7.1 "Myelin-specific proteins: a structurally diverse group of membrane-interacting molecules". BioFactors 39 (3): 233–41. January 2013. doi:10.1002/biof.1076. PMID 23780694.
- ↑ Jump up to: 8.0 8.1 8.2 Kamholz, John A.; Brucal, Michelle; Li, Jun; Shy, Michael (2007), "Myelin Protein Zero and CMT1B: A Tale of Two Phenotypes", Molecular Neurology (Elsevier): pp. 463–474, doi:10.1016/b978-012369509-3.50031-7, ISBN 9780123695093
- ↑ "Myelin protein zero exists as dimers and tetramers in native membranes of Xenopus laevis peripheral nerve". Journal of Neuroscience Research 67 (6): 766–71. March 2002. doi:10.1002/jnr.10167. PMID 11891790.
- ↑ Jump up to: 10.0 10.1 10.2 "Complete amino acid sequence of PO protein in bovine peripheral nerve myelin". The Journal of Biological Chemistry 262 (9): 4208–14. March 1987. doi:10.1016/S0021-9258(18)61334-1. PMID 2435734.
- ↑ "Membrane adhesion in peripheral myelin: good and bad wraps with protein P0". Structure 4 (11): 1239–44. November 1996. doi:10.1016/s0969-2126(96)00132-3. PMID 8939762.
- ↑ Jump up to: 12.0 12.1 "Peripheral myelin protein 22 and protein zero: a novel association in peripheral nervous system myelin". The Journal of Neuroscience 19 (9): 3396–403. May 1999. doi:10.1523/JNEUROSCI.19-09-03396.1999. PMID 10212299.
- ↑ Pareyson, Davide; Marchesi, Chiara; Salsano, Ettore (2013), "Dominant Charcot–Marie–Tooth syndrome and cognate disorders", Peripheral Nerve Disorders, Handbook of Clinical Neurology, 115, Elsevier, pp. 817–845, doi:10.1016/b978-0-444-52902-2.00047-3, ISBN 9780444529022, PMID 23931817
Further reading
- "Charcot-Marie-Tooth disease: a new paradigm for the mechanism of inherited disease". Trends in Genetics 10 (4): 128–33. April 1994. doi:10.1016/0168-9525(94)90214-3. PMID 7518101.
- "Molecular Genetics of Charcot-Marie-Tooth Neuropathy". Advances in Human Genetics. 22. 1994. pp. 117–52. doi:10.1007/978-1-4757-9062-7_3. ISBN 978-1-4757-9064-1.
- "Mutations in the peripheral myelin genes and associated genes in inherited peripheral neuropathies". Human Mutation 13 (1): 11–28. 1999. doi:10.1002/(SICI)1098-1004(1999)13:1<11::AID-HUMU2>3.0.CO;2-A. PMID 9888385.
- "Corticosteroid- responsive asymmetric neuropathy with a myelin protein zero gene mutation". Neurology 59 (5): 767–9. September 2002. doi:10.1212/wnl.59.5.767. PMID 12221176.
- "Demyelinating and axonal features of Charcot-Marie-Tooth disease with mutations of myelin-related proteins (PMP22, MPZ and Cx32): a clinicopathological study of 205 Japanese patients". Brain 126 (Pt 1): 134–51. January 2003. doi:10.1093/brain/awg012. PMID 12477701.
- "Peripheral neuropathies caused by mutations in the myelin protein zero". Journal of the Neurological Sciences 242 (1–2): 55–66. March 2006. doi:10.1016/j.jns.2005.11.015. PMID 16414078.
- "Isolation and sequence determination of cDNA encoding the major structural protein of human peripheral myelin". Biochemical and Biophysical Research Communications 180 (2): 515–8. October 1991. doi:10.1016/S0006-291X(05)81094-0. PMID 1719967.
- "The hypertrophic forms of hereditary motor and sensory neuropathy. A study of hypertrophic Charcot-Marie-Tooth disease (HMSN type I) and Dejerine-Sottas disease (HMSN type III) in childhood". Brain 110 ( Pt 1) (1): 121–48. February 1987. doi:10.1093/brain/110.1.121. PMID 3467805.
- "Two cases of congenital hypomyelination neuropathy". Brain & Development 6 (6): 560–5. 1985. doi:10.1016/s0387-7604(84)80101-1. PMID 6099985.
- "Structure and chromosomal localization of the gene encoding the human myelin protein zero (MPZ)". Genomics 17 (3): 755–8. September 1993. doi:10.1006/geno.1993.1400. PMID 7503936.
- "Myelin protein zero gene mutated in Charcot-Marie-tooth type 1B patients". Proceedings of the National Academy of Sciences of the United States of America 90 (22): 10856–60. November 1993. doi:10.1073/pnas.90.22.10856. PMID 7504284. Bibcode: 1993PNAS...9010856S.
- "New mutation of the myelin P0 gene in a pedigree of Charcot-Marie-Tooth neuropathy 1". Biochemistry and Molecular Biology International 31 (1): 169–73. September 1993. PMID 7505151.
- "De novo mutation of the myelin P0 gene in Dejerine-Sottas disease (hereditary motor and sensory neuropathy type III)". Nature Genetics 5 (3): 266–8. November 1993. doi:10.1038/ng1193-266. PMID 7506095.
- "The major peripheral myelin protein zero gene: structure and localization in the cluster of Fc gamma receptor genes on human chromosome 1q21.3-q23". Human Molecular Genetics 2 (12): 2051–4. December 1993. doi:10.1093/hmg/2.12.2051. PMID 7509228.
- "Tomaculous neuropathy in chromosome 1 Charcot-Marie-Tooth syndrome". Acta Neuropathologica 87 (1): 91–7. 1994. doi:10.1007/BF00386259. PMID 7511317.
- "Rapid screening of myelin genes in CMT1 patients by SSCP analysis: identification of new mutations and polymorphisms in the P0 gene". Human Genetics 94 (6): 653–7. December 1994. doi:10.1007/bf00206959. PMID 7527371.
- "Myelin P0 glycoprotein: identification of the site phosphorylated in vitro and in vivo by endogenous protein kinases". Journal of Neurochemistry 64 (2): 902–7. February 1995. doi:10.1046/j.1471-4159.1995.64020902.x. PMID 7530295.
- "Identification of a de novo insertional mutation in P0 in a patient with a Déjérine-Sottas syndrome (DSS) phenotype". Human Molecular Genetics 3 (9): 1701–2. September 1994. doi:10.1093/hmg/3.9.1701. PMID 7530550.
- "Mutations in the myelin protein zero gene associated with Charcot-Marie-Tooth disease type 1B". Human Mutation 6 (1): 50–4. 1995. doi:10.1002/humu.1380060110. PMID 7550231.
External links
- GeneReviews/NCBI/NIH/UW entry on Charcot-Marie-Tooth Neuropathy Type 1
- GeneReviews/NCBI/NIH/UW entry on Charcot-Marie-Tooth Neuropathy Type 2
- Myelin+protein+zero at the US National Library of Medicine Medical Subject Headings (MeSH)
- Overview of all the structural information available in the PDB for UniProt: P25189 (Myelin protein P0) at the PDBe-KB.
 |



