Physics:Orange carotenoid protein
| Orange carotenoid-binding protein (Kerfeld et al., Structure 2003) | |||||||
|---|---|---|---|---|---|---|---|
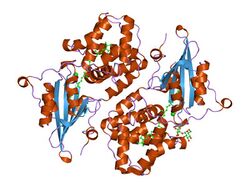 Crystal structure of orange carotenoid protein (Kerfeld et al., Structure 2003) | |||||||
| Identifiers | |||||||
| Organism | |||||||
| Symbol | Ocp | ||||||
| Alt. symbols | slr1963 | ||||||
| PDB | 1M98 (ECOD) | ||||||
| UniProt | P83689 | ||||||
| |||||||
| Pfam domains Carot_N, NTF2 | |||||||
Orange carotenoid protein (OCP) is a water-soluble protein which plays a role in photoprotection in diverse cyanobacteria.[1] It is the only photoactive protein known to use a carotenoid as the photoresponsive chromophore. The protein consists of two domains, with a single keto-carotenoid molecule non-covalently bound between the two domains. It is a very efficient quencher of excitation energy absorbed by the primary light-harvesting antenna complexes of cyanobacteria, the phycobilisomes. The quenching is induced by blue-green light. It is also capable of preventing oxidative damage by directly scavenging singlet oxygen (1O2).
History
OCP was first described in 1981 by Holt and Krogmann[2] who isolated it from the unicellular cyanobacterium Arthrospira maxima,[3][4] although its function would remain obscure until 2006. The crystal structure of the OCP was reported in 2003.[5] At the same time the protein was shown to be an effective quencher of singlet oxygen and was suggested to be involved in photoprotection, or carotenoid transport.[6][7][8] In 2000, it was demonstrated that cyanobacteria could perform photoprotective fluorescence quenching independent of lipid phase transitions, differential transmembrane pH, and inhibitors.[9] The action spectrum for this quenching process suggested the involvement of carotenoids,[10] and the specific involvement of the OCP was later demonstrated by Kirilovsky and coworkers in 2006.[11] In 2008, OCP was shown to require photoactivation by strong blue-green light for its photoprotective quenching function.[12] Photoactivation is accompanied by a pronounced color change, from orange to red, which had been previously observed by Kerfeld et al in the initial structural studies.[7][8][5] In 2015 a combination of biophysical methods by researchers in Berkeley showed that the visible color change is the consequence of a 12Å translocation of the carotenoid.[13][14][15]
Physiological significance
For a long time, cyanobacteria were considered incapable of performing non-photochemical quenching (NPQ) as a photoprotective mechanism, relying instead on a mechanism of energy redistribution between the two photosynthetic reaction centers, PSII and PSI, known as "state transitions".[16]
OCP is found in a majority of cyanobacterial genomes,[1][17] with remarkable conservation of its amino acid sequence, implying evolutionary constraints to preserve an important function. Mutant cells engineered to lack OCP photobleach under high light[11] and become photoinhibited more rapidly under fluctuating light.[18] Under nutrient stress conditions, which are expected to be norm in marine environments, photoprotective mechanisms such as OCP become important even at lower irradiances.[19]
This protein is not found in chloroplasts, and appears to be specific to cyanobacteria.[20]
Function
Photoactivity
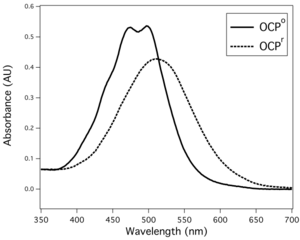
Upon illumination with blue-green light, OCP switches from an orange form (OCPO) to a red form (OCPR). The reversion of OCPR to OCPO is light independent and occurs slowly in darkness. OCPO is considered the dark, stable form of the protein, and does not contribute to phycobilisome quenching. OCPR is considered to be essential for induction of the photoprotection mechanism. The photoconversion from the orange to red form has a poor light efficiency (very low quantum yield), which helps to ensure the protein's photoprotective role only functions during high light conditions; otherwise, the dissipative NPQ process could unproductively divert light energy away from photosynthesis under light-limiting conditions.[12][15]
Energy quenching
As evidenced by a decreased fluorescence, OCP in its red form is capable of dissipating absorbed light energy from the phycobilisome antenna complex. According to Rakhimberdieva and coworkers, about 30-40% of the energy absorbed by phycobilisomes does not reach the reaction centers when the carotenoid-induced NPQ is active.[21] The exact mechanism and quenching site in both the carotenoid as well as the phycobilisome still remain uncertain. The linker polypeptide ApcE in the allophycocyanin (APC) core of the phycobilisomes is known to be important,[11][22] but is not the site of quenching.[23] Several lines of evidence suggest that it is the 660 nm fluorescence emission band of the APC core which is quenched by OCPR.[21][23][24] The temperature dependence of the rate of fluorescence quenching is similar to that of soluble protein folding,[25] supporting the hypothesis that OCPO slightly unfolds when it converts to OCPR.
Singlet oxygen quenching
As first shown in 2003,[5] the auxiliary function of carotenoids as quenchers of singlet oxygen contributes to the photoprotective role of OCP has also been demonstrated under strong orange-red light, which are conditions where OCP cannot be photoactivated for its energy-quenching role.[26] This is significant because all oxygenic phototrophs have a particular risk of oxidative damage initiated by singlet oxygen (1O2), which is produced when their own light-harvesting pigments act as photosensitizers.[27]
Structure

3D structure
The three-dimensional protein structure of OCP (in the OCPO form) was solved in 2003, before its photoprotective role had been defined.[6] The 35 kDa protein contains two structural domains: an all-α-helical N-terminal domain (NTD) consisting of two interleaved 4-helix bundles, and a mixed α/β C-terminal domain (CTD). The two domains are connected by an extended linker. In OCPO, the carotenoid spans both domains, which are tightly associated in this form of protein. In 2013 Kerfeld and co-workers showed that the NTD is the effector (quencher) domain of the protein while the CTD plays a regulatory role.[28]
Protein–protein interactions
The OCP participates in key protein–protein interactions that are critical to its photoprotective function. The activated OCPR form binds to allophycocyanin in the core of the phycobilisome and initiates the OCP-dependent photoprotective quenching mechanism. Another protein, the fluorescence recovery protein (FRP), interacts with the CTD in OCPR and catalyzes the reaction which reverts it back to the OCPO form.[29] Because OCPO cannot bind to the phycobilisome antenna, FRP effectively can detach OCP from the antenna and restore full light-harvesting capacity.
Evolution
The primary structure (amino acid sequence) is highly conserved among OCP sequences, and the full-length protein is usually co-located on the chromosome with a second open reading frame[7][8] that was later characterized as the FRP.[1] Often, biosynthetic genes for ketocarotenoid synthesis (e.g., CrtW) are nearby. These conserved functional linkages underscore the evolutionary importance of the OCP style of photoprotection for many cyanobacteria.
The first structure determination of the OCP coincided with the beginning of the genome sequencing era, and it was already apparent in 2003 that there is also a variety of evolutionarily related genes which encode proteins with only one of the two domains present in OCP.[5][7][8] The N-terminal domain (NTD), "Carot N", is found only in cyanobacteria, but exhibits a considerable amount of gene duplication. The C-terminal domain (CTD), however, is homologous with the widespread NTF2 superfamily, which shares a protein fold with its namesake, nuclear transport factor 2, as well as around 20 other subfamilies of proteins with functions as diverse as limonene-1,2-epoxide hydrolase, SnoaL polyketide cyclase, and delta-5-3-ketosteroid isomerase (KSI). Most, if not all, of the members of the NTF2 superfamily form oligomers, often using the surface of their beta sheet to interact with another monomer or other protein.
Bioinformatic analyses carried out over the past 15 years has resulted in the identification of new groups of carotenoid proteins:[30] In addition to new families of the OCP,[17] there are HCPs[31] and CCPs that correspond to the NTD and CTD of the OCP, respectively. Based on the primary structure, the HCPs can be subdivided into at least nine evolutionarily distinct clades, each binds carotenoid.[32][33] The CCPs resolve into 2 major groups, and these proteins also bind carotenoid.[34] Given these data, and the ability to devolve OCP into its two component domains while retaining function[35] has led to a reconstruction of the evolution of the OCP.[36][35]
Applications
Its water-solubility, together with its status as the only known photoactive protein containing a carotenoid, makes the OCP a valuable model for studying solution-state energetic and photophysical properties of carotenoids, which are a diverse class of molecules found across all domains of life. Moreover, carotenoids are widely investigated for their properties as anti-oxidants, and thus the protein may serve as a template for delivery of carotenoids for therapeutic purposes in human medicine.
Because of its high efficiency of fluorescence quenching, coupled to its low quantum yield of photoactivation by specific wavelengths of light, OCP has ideal properties as a photoswitch and has been proposed as a novel system for developing optogenetics technologies [1] and may have other applications in optofluidics and biophotonics.
See also
- Photoprotection
- Xanthophylls
- Biological pigments
- Orange carotenoid N-terminal domain
- Phycobilisome
- Fluorescence recovery protein
- Photosynthetic state transition
- Ketocarotenoids
References
- ↑ 1.0 1.1 1.2 1.3 "The Orange Carotenoid Protein: a blue-green light photoactive protein". Photochemical & Photobiological Sciences 12 (7): 1135–43. July 2013. doi:10.1039/c3pp25406b. PMID 23396391.
- ↑ "David W. Krogmann, 1931-2016". Photosynthesis Research 132 (1): 1–12. April 2017. doi:10.1007/s11120-016-0335-x. PMID 28155215.
- ↑ "A carotenoid-protein from cyanobacteria". Biochimica et Biophysica Acta (BBA) - Bioenergetics 637 (3): 408–414. 1981. doi:10.1016/0005-2728(81)90045-1. ISSN 0005-2728.
- ↑ "David W. Krogmann: 1934-2016". ASPB News 43 (4): 25–27. http://cl.exct.net/?qs=9ad0899c8127e3ffa91a45fde476c74eaedbfa6dacf78403c047fd26ac1b3bbc4138dd6ef8e1ca52.
- ↑ 5.0 5.1 5.2 5.3 "The Crystal Structure of a Cyanobacterial Water-Soluble Carotenoid Binding Protein" (in en). Structure 11 (1): 55–65. January 2003. doi:10.1016/S0969-2126(02)00936-X. PMID 12517340.
- ↑ 6.0 6.1 "The crystal structure of a cyanobacterial water-soluble carotenoid binding protein". Structure 11 (1): 55–65. January 2003. doi:10.1016/S0969-2126(02)00936-X. PMID 12517340.
- ↑ 7.0 7.1 7.2 7.3 "Structure and Function of the Water-Soluble Carotenoid-Binding Proteins of Cyanobacteria". Photosynthesis Research 81 (3): 215–225. 2004. doi:10.1023/b:pres.0000036886.60187.c8. ISSN 0166-8595. PMID 16034528.
- ↑ 8.0 8.1 8.2 8.3 "Water-soluble carotenoid proteins of cyanobacteria". Archives of Biochemistry and Biophysics 430 (1): 2–9. October 2004. doi:10.1016/j.abb.2004.03.018. PMID 15325905. https://escholarship.org/content/qt3dm533x9/qt3dm533x9.pdf?t=lnoslm.
- ↑ "Photosystem II fluorescence quenching in the cyanobacterium Synechocystis PCC 6803: involvement of two different mechanisms". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1457 (3): 229–42. April 2000. doi:10.1016/S0005-2728(00)00104-3. PMID 10773167.
- ↑ "Carotenoid-induced quenching of the phycobilisome fluorescence in photosystem II-deficient mutant of Synechocystis sp". FEBS Letters 574 (1–3): 85–8. September 2004. doi:10.1016/j.febslet.2004.07.087. PMID 15358544.
- ↑ 11.0 11.1 11.2 "A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria". The Plant Cell 18 (4): 992–1007. April 2006. doi:10.1105/tpc.105.040121. PMID 16531492.
- ↑ 12.0 12.1 "A photoactive carotenoid protein acting as light intensity sensor". Proceedings of the National Academy of Sciences of the United States of America 105 (33): 12075–80. August 2008. doi:10.1073/pnas.0804636105. PMID 18687902. Bibcode: 2008PNAS..10512075W.
- ↑ "PHOTOSYNTHESIS. A 12 Å carotenoid translocation in a photoswitch associated with cyanobacterial photoprotection". Science 348 (6242): 1463–6. June 2015. doi:10.1126/science.aaa7234. PMID 26113721.
- ↑ "Local and global structural drivers for the photoactivation of the orange carotenoid protein". Proceedings of the National Academy of Sciences of the United States of America 112 (41): E5567-74. October 2015. doi:10.1073/pnas.1512240112. PMID 26385969. Bibcode: 2015PNAS..112E5567G.
- ↑ 15.0 15.1 "Orange is the New Red" (in en). Lawrence Berkeley National Laboratory Press release. LegiStorm. https://www.legistorm.com/stormfeed/view_rss/671624/organization/107078/title/orange-is-the-new-red-lawrence-berkeley-national-laboratory-press-release-legistorm.html.
- ↑ "Regulation of excitation energy transfer in organisms containing phycobilins". Photosynthesis Research 20 (1): 1–34. April 1989. doi:10.1007/BF00028620. PMID 24425462.
- ↑ 17.0 17.1 "Additional families of orange carotenoid proteins in the photoprotective system of cyanobacteria". Nature Plants 3 (8): 17089. July 2017. doi:10.1038/nplants.2017.89. PMID 28692021.
- ↑ "Occurrence and function of the orange carotenoid protein in photoprotective mechanisms in various cyanobacteria". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1777 (10): 1344–54. October 2008. doi:10.1016/j.bbabio.2008.07.002. PMID 18694721.
- ↑ "Light-induced energy dissipation in iron-starved cyanobacteria: roles of OCP and IsiA proteins". The Plant Cell 19 (2): 656–72. February 2007. doi:10.1105/tpc.106.045351. PMID 17307930.
- ↑ "Structure, Diversity, and Evolution of a New Family of Soluble Carotenoid-Binding Proteins in Cyanobacteria". Molecular Plant 9 (10): 1379–1394. October 2016. doi:10.1016/j.molp.2016.06.009. PMID 27392608.
- ↑ 21.0 21.1 "Carotenoid-triggered energy dissipation in phycobilisomes of Synechocystis sp. PCC 6803 diverts excitation away from reaction centers of both photosystems". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1797 (2): 241–9. February 2010. doi:10.1016/j.bbabio.2009.10.008. PMID 19879235.
- ↑ "Phycobilin/chlorophyll excitation equilibration upon carotenoid-induced non-photochemical fluorescence quenching in phycobilisomes of the cyanobacterium Synechocystis sp. PCC 6803". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1767 (6): 757–65. June 2007. doi:10.1016/j.bbabio.2006.12.007. PMID 17240350.
- ↑ 23.0 23.1 "ApcD, ApcF and ApcE are not required for the Orange Carotenoid Protein related phycobilisome fluorescence quenching in the cyanobacterium Synechocystis PCC 6803". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1817 (8): 1418–27. August 2012. doi:10.1016/j.bbabio.2011.11.020. PMID 22172739.
- ↑ "Effect of APCD and APCF subunits depletion on phycobilisome fluorescence of the cyanobacterium Synechocystis PCC 6803". Journal of Photochemistry and Photobiology B: Biology 133: 153–60. April 2014. doi:10.1016/j.jphotobiol.2014.03.012. PMID 24727864.
- ↑ "Protein-protein interactions in carotenoid triggered quenching of phycobilisome fluorescence in Synechocystis sp. PCC 6803". FEBS Letters 581 (13): 2429–33. May 2007. doi:10.1016/j.febslet.2007.04.056. PMID 17485085.
- ↑ "The Cyanobacterial Photoactive Orange Carotenoid Protein Is an Excellent Singlet Oxygen Quencher". The Plant Cell 26 (4): 1781–1791. April 2014. doi:10.1105/tpc.114.123802. PMID 24748041.
- ↑ "Singlet oxygen production in photosystem II and related protection mechanism". Photosynthesis Research 98 (1–3): 551–64. 2008. doi:10.1007/s11120-008-9349-3. PMID 18780159.
- ↑ "Structural and functional modularity of the orange carotenoid protein: distinct roles for the N- and C-terminal domains in cyanobacterial photoprotection". The Plant Cell 26 (1): 426–37. January 2014. doi:10.1105/tpc.113.118588. PMID 24399299.
- ↑ "Crystal structure of the FRP and identification of the active site for modulation of OCP-mediated photoprotection in cyanobacteria". Proceedings of the National Academy of Sciences of the United States of America 110 (24): 10022–7. June 2013. doi:10.1073/pnas.1303673110. PMID 23716688. Bibcode: 2013PNAS..11010022S.
- ↑ "Structure and functions of Orange Carotenoid Protein homologs in cyanobacteria". Current Opinion in Plant Biology 37: 1–9. June 2017. doi:10.1016/j.pbi.2017.03.010. PMID 28391046.
- ↑ "Structure, Diversity, and Evolution of a New Family of Soluble Carotenoid-Binding Proteins in Cyanobacteria". Molecular Plant 9 (10): 1379–1394. October 2016. doi:10.1016/j.molp.2016.06.009. PMID 27392608.
- ↑ "Excited-State Properties of Canthaxanthin in Cyanobacterial Carotenoid-Binding Proteins HCP2 and HCP3". The Journal of Physical Chemistry B 124 (24): 4896–4905. June 2020. doi:10.1021/acs.jpcb.0c03137. PMID 32437153.
- ↑ "Structural and spectroscopic characterization of HCP2". Biochimica et Biophysica Acta (BBA) - Bioenergetics 1860 (5): 414–424. May 2019. doi:10.1016/j.bbabio.2019.03.004. PMID 30880081.
- ↑ "Structural analysis of a new carotenoid-binding protein: the C-terminal domain homolog of the OCP". Scientific Reports 10 (1): 15564. September 2020. doi:10.1038/s41598-020-72383-y. PMID 32968135. Bibcode: 2020NatSR..1015564D.
- ↑ 35.0 35.1 "Synthetic OCP heterodimers are photoactive and recapitulate the fusion of two primitive carotenoproteins in the evolution of cyanobacterial photoprotection". The Plant Journal 91 (4): 646–656. August 2017. doi:10.1111/tpj.13593. PMID 28503830.
- ↑ "Structure, function and evolution of the cyanobacterial orange carotenoid protein and its homologs". The New Phytologist 215 (3): 937–951. August 2017. doi:10.1111/nph.14670. PMID 28675536.
 |