Chemistry:Singlet oxygen
| |
| |
| Names | |
|---|---|
| IUPAC name
Singlet oxygen
| |
| Systematic IUPAC name
Dioxidene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| 491 | |
| |
| |
| Properties | |
| O2 | |
| Molar mass | 31.998 g·mol−1 |
| Reacts | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with two oxygen atoms in a quantum state where all electrons are spin-paired, known as a singlet state. It is the lowest excited state of the diatomic oxygen molecule, which in general has the chemical structure O=O and chemical formula O2. Singlet oxygen can be written more specifically as 1[O2] or 1O2. The more prevalent ground state of O2 is known as triplet oxygen. At room temperature, singlet oxygen will slowly decay into triplet oxygen, releasing the energy of excitation.
Singlet oxygen is a gas with physical properties differing only subtly from the ground state. In terms of its chemical reactivity, however, singlet oxygen is far more reactive toward organic compounds. It is responsible for the photodegradation of many materials but can be put to constructive use in preparative organic chemistry and photodynamic therapy. Trace amounts of singlet oxygen are found in the upper atmosphere and in polluted urban atmospheres where it contributes to the formation of lung-damaging nitrogen dioxide.[1]: 355–68 It often appears and coexists confounded in environments that also generate ozone, such as pine forests with photodegradation of turpentine. The terms "singlet oxygen" and "triplet oxygen" derive from each form's number of electron spins. The singlet has only one possible arrangement of electron spins with a total quantum spin of 0, while the triplet has three possible arrangements of electron spins with a total quantum spin of 1, corresponding to three degenerate states.
In spectroscopic notation, the lowest singlet and triplet forms of O2 are labeled 1Δg and 3Σ−g, respectively.[2][3][4]
Electronic structure
Singlet oxygen refers to one of two singlet electronic excited states. The two singlet states are denoted 1Σ+g and 1Δg (the preceding superscript "1" indicates a singlet state). The singlet states of oxygen are 158 and 95 kilojoules per mole higher in energy than the triplet ground state of oxygen. Under most common laboratory conditions, the higher energy 1Σ+g singlet state rapidly converts to the more stable, lower energy 1Δg singlet state.[2] This more stable of the two excited states has its two valence electrons spin-paired in one π* orbital while the second π* orbital is empty. This state is referred to by the title term, singlet oxygen, commonly abbreviated 1O2, to distinguish it from the triplet ground state molecule, 3O2.[2][3]
Molecular orbital theory predicts the electronic ground state denoted by the molecular term symbol 3Σ–g, and two low-lying excited singlet states with term symbols 1Δg and 1Σ+g. These three electronic states differ only in the spin and the occupancy of oxygen's two antibonding πg-orbitals, which are degenerate (equal in energy). These two orbitals are classified as antibonding and are of higher energy. Following Hund's first rule, in the ground state, these electrons are unpaired and have like (same) spin. This open-shell triplet ground state of molecular oxygen differs from most stable diatomic molecules, which have singlet (1Σ+g) ground states.[5]
Two less stable, higher energy excited states are readily accessible from this ground state, again in accordance with Hund's first rule;[6] the first moves one of the high energy unpaired ground state electrons from one degenerate orbital to the other, where it "flips" and pairs the other, and creates a new state, a singlet state referred to as the 1Δg state (a term symbol, where the preceding superscripted "1" indicates it as a singlet state).[2][3] Alternatively, both electrons can remain in their degenerate ground state orbitals, but the spin of one can "flip" so that it is now opposite to the second (i.e., it is still in a separate degenerate orbital, but no longer of like spin); this also creates a new state, a singlet state referred to as the 1Σ+g state.[2][3] The ground and first two singlet excited states of oxygen can be described by the simple scheme in the figure below.[7][8]
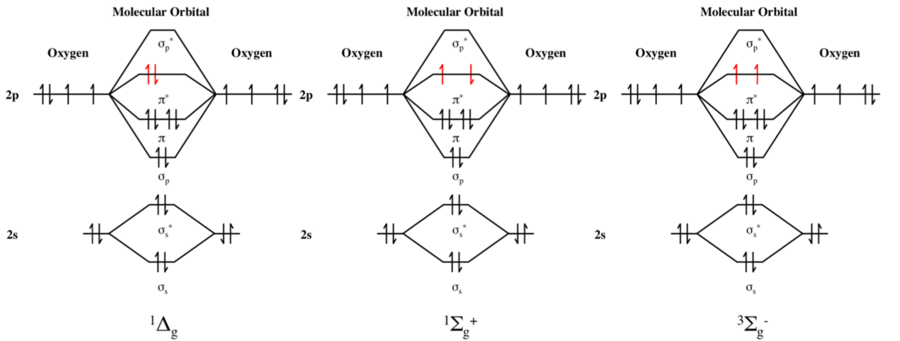
The 1Δg singlet state is 7882.4 cm−1 above the triplet 3Σ−g ground state.,[3][9] which in other units corresponds to 94.29 kJ/mol or 0.9773 eV. The 1Σ+g singlet is 13 120.9 cm−1[3][9] (157.0 kJ/mol or 1.6268 eV) above the ground state.
Radiative transitions between the three low-lying electronic states of oxygen are formally forbidden as electric dipole processes.[10] The two singlet-triplet transitions are forbidden both because of the spin selection rule ΔS = 0 and because of the parity rule that g-g transitions are forbidden.[11] The singlet-singlet transition between the two excited states is spin-allowed but parity-forbidden.
The lower, O2(1Δg) state is commonly referred to as singlet oxygen. The energy difference of 94.3 kJ/mol between ground state and singlet oxygen corresponds to a forbidden singlet-triplet transition in the near-infrared at ~1270 nm.[12] As a consequence, singlet oxygen in the gas phase is relatively long lived (54-86 milliseconds),[13] although interaction with solvents reduces the lifetime to microseconds or even nanoseconds.[14] In 2021, the lifetime of airborne singlet oxygen at air/solid interfaces was measured to be 550 microseconds.[15]
The higher 1Σ+g state is moderately short lived. In the gas phase, it relaxes primarily to the ground state triplet with a mean lifetime of 11.8 seconds.[10] However in solvents such as CS2 and CCl4, it relaxes to the lower singlet 1Δg in milliseconds due to radiationless decay channels.[10]
Paramagnetism due to orbital angular momentum
Both singlet oxygen states have no unpaired electrons and therefore no net electron spin. The 1Δg is however paramagnetic as shown by the observation of an electron paramagnetic resonance (EPR) spectrum.[16][17][18] The paramagnetism of the 1Δg state is due to a net orbital (and not spin) electronic angular momentum. In a magnetic field the degeneracy of the levels is split into two levels with z projections of angular momenta +1ħ and −1ħ around the molecular axis. The magnetic transition between these levels gives rise to the EPR transition.
Production
Various methods for the production of singlet oxygen exist. Irradiation of oxygen gas in the presence of an organic dye as a sensitizer, such as rose bengal, methylene blue, or porphyrins—a photochemical method—results in its production.[19][9] Large steady state concentrations of singlet oxygen are reported from the reaction of triplet excited state pyruvic acid with dissolved oxygen in water.[20] Singlet oxygen can also be produced by chemical procedures without irradiation. One chemical method involves the decomposition of triethylsilyl hydrotrioxide generated in situ from triethylsilane and ozone.[21]
- (C2H5)3SiH + O3 → (C2H5)3SiOOOH → (C2H5)3SiOH + O2(1Δg)
Another method uses a reaction of hydrogen peroxide with sodium hypochlorite in aqueous solution:[19]
- H2O2 + NaOCl → O2(1Δg) + NaCl + H2O
A retro-Diels Alder reaction of the diphenylanthracene peroxide can also yield singlet oxygen, along with an diphenylanthracene:[22]
A third method liberates singlet oxygen via phosphite ozonides, which are, in turn, generated in situ such as triphenyl phosphite ozonide.[23][24] Phosphite ozonides will decompose to give singlet oxygen:[25]
- (RO)3P + O3 → (RO)3PO3
- (RO)3PO3 → (RO)3PO + O2(1Δg)
An advantage of this method is that it is amenable to non-aqueous conditions.[25]
Potassium tetraperoxochromate(V) decomposes in water (but not neat) to give singlet oxygen. However, the resulting potassium chromate can also oxidize sensitive substrates.[26]
Reactions
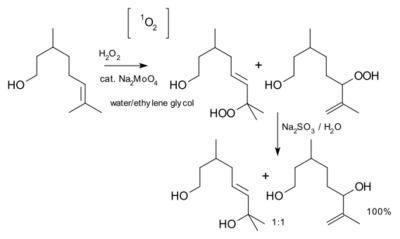
Because of differences in their electron shells, singlet and triplet oxygen differ in their chemical properties; singlet oxygen is highly reactive.[27] The lifetime of singlet oxygen depends on the medium and pressure. In normal organic solvents, the lifetime is only a few microseconds whereas in solvents lacking C-H bonds, the lifetime can be as long as seconds.[25][28]
Unlike ground state oxygen, singlet oxygen participates in Diels–Alder [4+2]- and [2+2]-cycloaddition reactions and formal concerted ene reactions (Schenck ene reaction), causing photooxygenation.[25] It oxidizes thioethers to sulfoxides. Organometallic complexes are often degraded by singlet oxygen.[29][30] With some substrates 1,2-dioxetanes are formed; cyclic dienes such as 1,3-cyclohexadiene form [4+2] cycloaddition adducts.[31]
The [4+2]-cycloaddition between singlet oxygen and furans is widely used in organic synthesis.[32][33]
In singlet oxygen reactions with alkenic allyl groups, e.g., citronella, shown, by abstraction of the allylic proton, in an ene-like reaction, yielding the allyl hydroperoxide, R–O–OH (R = alkyl), which can then be reduced to the corresponding allylic alcohol.[25][34][35][36]
Analytical and physical chemistry

Singlet oxygen luminesces concomitant with its decay to the triplet ground state. This phenomenon was first observed in the thermal degradation of the endo peroxide of rubrene.[37]
Biochemistry
In photosynthesis, singlet oxygen can be produced from the light-harvesting chlorophyll molecules. One of the roles of carotenoids in photosynthetic systems is to prevent damage caused by produced singlet oxygen by either removing excess light energy from chlorophyll molecules or quenching the singlet oxygen molecules directly.

In mammalian biology, singlet oxygen is one of the reactive oxygen species, which is linked to oxidation of LDL cholesterol and resultant cardiovascular effects. Polyphenol antioxidants can scavenge and reduce concentrations of reactive oxygen species and may prevent such deleterious oxidative effects.[39]
Ingestion of pigments capable of producing singlet oxygen with activation by light can produce severe photosensitivity of skin (see phototoxicity, photosensitivity in humans, photodermatitis, phytophotodermatitis). This is especially a concern in herbivorous animals (see Photosensitivity in animals), and has been harnessed for medical uses in photodynamic therapy.
References
- ↑ "Singlet Molecular Oxygen". Advances in Photochemistry. 7. 1969. pp. 311–71. doi:10.1002/9780470133378.ch4. ISBN 9780470133378.
- ↑ 2.0 2.1 2.2 2.3 2.4 Klán, Petr; Wirz, Jakob (2009). Photochemistry of Organic Compounds: From Concepts to Practice (Repr. 2010 ed.). Chichester, West Sussex, U.K.: Wiley. ISBN 978-1405190886.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Atkins, Peter; de Paula, Julio (2006). Atkins' Physical Chemistry (8th ed.). W.H.Freeman. pp. 482–3. ISBN 978-0-7167-8759-4. https://archive.org/details/atkinsphysicalch00pwat/page/482.
- ↑ Hill, Christian. "Molecular Term Symbols". http://www.christianhill.co.uk/static/teaching/cribs/molecular_term_symbols.pdf.
- ↑ Levine, Ira N. (1991). Quantum Chemistry (4th ed.). Prentice-Hall. p. 383. ISBN 978-0-205-12770-2.
- ↑ Frimer, Aryeh A. (1985). Singlet Oxygen: Volume I, Physical-Chemical Aspects. Boca Raton, Fla.: CRC Press. pp. 4–7. ISBN 9780849364396.
- ↑ For triplet ground state on right side of diagram, see C.E.Housecroft and A.G.Sharpe Inorganic Chemistry, 2nd ed. (Pearson Prentice-Hall 2005), p.35 ISBN 0130-39913-2
- ↑ For changes in singlet states on left and in centre, see F. Albert Cotton and Geoffrey Wilkinson. Advanced Inorganic Chemistry, 5th ed. (John Wiley 1988), p.452 ISBN 0-471-84997-9
- ↑ 9.0 9.1 9.2 "Physical Mechanisms of Generation and Deactivation of Singlet Oxygen". Chemical Reviews 103 (5): 1685–757. May 2003. doi:10.1021/cr010371d. PMID 12744692.
- ↑ 10.0 10.1 10.2 Weldon, Dean; Poulsen, Tina D.; Mikkelsen, Kurt V.; Ogilby, Peter R. (1999). "Singlet Sigma: The "Other" Singlet Oxygen in Solution". Photochemistry and Photobiology 70 (4): 369–379. doi:10.1111/j.1751-1097.1999.tb08238.x.
- ↑ Thomas Engel; Philip Reid (2006). Physical Chemistry. PEARSON Benjamin Cummings. p. 580. ISBN 978-0-8053-3842-3.
- ↑ Guy P. Brasseur; Susan Solomon (January 15, 2006). Aeronomy of the Middle Atmosphere: Chemistry and Physics of the Stratosphere and Mesosphere. Springer Science & Business Media. pp. 220–. ISBN 978-1-4020-3824-2. https://books.google.com/books?id=Z5OtlDjfXkkC&pg=PA220.
- ↑ Physical Mechanisms of Generation and Deactivation of Singlet Oxygen Claude Schweitzer
- ↑ "Rate constants for the decay and reactions of the lowest electronically excited singlet state of molecular oxygen in solution. An expanded and revised compilation.". J. Phys. Chem. Ref. Data 24 (2): 663–677. 1995. doi:10.1063/1.555965. Bibcode: 1995JPCRD..24..663W.
- ↑ Andrés M. Durantini (2021). "Interparticle Delivery and Detection of Volatile Singlet Oxygen at Air/Solid Interfaces". Environmental Science & Technology 55 (6): 3559–3567. doi:10.1021/acs.est.0c07922. PMID 33660980. Bibcode: 2021EnST...55.3559D.
- ↑ Hasegawa, Keisuke; Yamada, Kenji; Sasase, Ryouji; Miyazaki, Ryota; Kikuchi, Azusa; Yagi, Mikio (2008). "Direct measurements of absolute concentration and lifetime of singlet oxygen in the gas phase by electron paramagnetic resonance". Chemical Physics Letters 457 (4): 312–314. doi:10.1016/j.cplett.2008.04.031. Bibcode: 2008CPL...457..312H.
- ↑ "Time-resolved EPR study of singlet oxygen in the gas phase". The Journal of Physical Chemistry A 117 (25): 5232–40. June 2013. doi:10.1021/jp403648d. PMID 23768193. Bibcode: 2013JPCA..117.5232R.
- ↑ Falick, AM (1965). "Paramagnetic resonance spectrum of the 1Δg oxygen molecule". J. Chem. Phys. 42 (5): 1837–1838. doi:10.1063/1.1696199. Bibcode: 1965JChPh..42.1837F.
- ↑ 19.0 19.1 "Christopher Spencer Foote's Discovery of the Role of Singlet Oxygen [1O2 (1Δg)] in Photosensitized Oxidation Reactions". Acc. Chem. Res. 39 (11): 797–804. 2006. doi:10.1021/ar050191g. PMID 17115719.
- ↑ "Production of Singlet Oxygen (1O2) during the Photochemistry of Aqueous Pyruvic Acid: The Effects of pH and Photon Flux under Steady-State O2(aq) Concentration". Environmental Science and Technology 53 (21): 12425–12432. September 2019. doi:10.1021/acs.est.9b03742. PMID 31550134. Bibcode: 2019EnST...5312425E.
- ↑ "Generation of 1Δg from triethylsilane and ozone". Journal of the American Chemical Society 108 (9): 2472–3. April 1986. doi:10.1021/ja00269a070. PMID 22175617.
- ↑ 裴, 坚 (2016). 基础有机化学 (4th ed.). 北京大学出版社. pp. 1072–1073. ISBN 978-7-301-27212-1.
- ↑ Bartlett, Paul D.; Mendenhall, G. David; Durham, Dana L. (October 1980). "Controlled generation of singlet oxygen at low temperatures from triphenyl phosphite ozonide" (in en). The Journal of Organic Chemistry 45 (22): 4269–4271. doi:10.1021/jo01310a001. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo01310a001.
- ↑ Housecroft, Catherine E.; Sharpe, Alan G. (2008). "Chapter 15: The group 16 elements". Inorganic Chemistry (3rd ed.). Pearson. p. 438f. ISBN 9780131755536. https://archive.org/details/inorganicchemist00hous_159.
- ↑ 25.0 25.1 25.2 25.3 25.4 Wasserman, Harry H.; DeSimone, Robert W.; Chia, Kristie R. X.; Banwell, Martin G. (2001). "Encyclopedia of Reagents for Organic Synthesis". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rs035. ISBN 978-0471936237.
- ↑ Peters, John W.; Bekowies, Paul J.; Winer, Arthur M.; Pitts, James N., Jr. (1975). "Potassium perchromate as a source of singlet oxygen" (in English). Journal of the American Chemical Society (ACS Publications) 97 (12): 3299–3306. doi:10.1021/ja00845a003.
- ↑ Ho, Raymond YN; Liebman, Joel F.; Valentine, Joan Selverstone (1995). "Overview of the Energetics and Reactivity of Oxygen". Active Oxygen in Chemistry. London: Blackie Academic & Professional. pp. 1–23. doi:10.1007/978-94-007-0874-7_1. ISBN 978-0-7514-0371-8.
- ↑ Kuntner N (2018). "Modeling and simulation of electronic excitation in oxygen-helium discharges and plasma-assisted combustion". University of Stuttgart. doi = http://dx.doi.org/10.18419/opus-9925
- ↑ Clennan, EL; Pace, A (2005). "Advances in singlet oxygen chemistry". Tetrahedron 61 (28): 6665–6691. doi:10.1016/j.tet.2005.04.017.
- ↑ "Singlet oxygen: there is indeed something new under the sun". Chemical Society Reviews 39 (8): 3181–209. August 2010. doi:10.1039/b926014p. PMID 20571680.
- ↑ Carey, Francis A.; Sundberg, Richard J. (1985). Structure and mechanisms (2 ed.). New York: Plenum Press. ISBN 978-0306411984.
- ↑ Montagnon, T.; Kalaitzakis, D.; Triantafyllakis, M.; Stratakis, M.; Vassilikogiannakis, G. (2014). "Furans and Singlet Oxygen - Why There Is More to Come from this Powerful Partnership". Chemical Communications 50 (98): 15480–15498. doi:10.1039/C4CC02083A. PMID 25316254. https://zenodo.org/record/32921.
- ↑ Ghogare, A.A.; Greer, A. (2016). "Using Singlet Oxygen to Synthesise Natural Products and Drugs". Chemical Reviews 116 (17): 9994–10034. doi:10.1021/acs.chemrev.5b00726. PMID 27128098.
- ↑ Stephenson, L. M.; Grdina, Mary Jo; Orfanopoulos, Michael (November 1980). "Mechanism of the ene reaction between singlet oxygen and olefins". Accounts of Chemical Research 13 (11): 419–425. doi:10.1021/ar50155a006.
- ↑ This reaction is not a true ene reaction, because it is not concerted; singlet oxygen forms an "epoxide oxide" exciplex, which then abstracts the hydrogen. See Alberti et al, op. cit.
- ↑ Alsters, Paul L.; Jary, Walther; Nardello-Rataj, Veronique; Jean-Marie, Aubry (2009). "Dark Singlet Oxygenation of β-Citronellol: A Key Step in the Manufacture of Rose Oxide". Organic Process Research & Development 14: 259–262. doi:10.1021/op900076g.
- ↑ Franz, Karl A.; Kehr, Wolfgang G.; Siggel, Alfred; Wieczoreck, Jürgen; Adam, Waldemar (2000). "Luminescent Materials". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a15_519. ISBN 3-527-30673-0.
- ↑ Devika, Sivakumar; Rakhi, Raju; Y.T., Kamal; Shahana, Salam (3 January 2023). Porphyrinoid Photosensitizers for Targeted and Precise Photodynamic Therapy: Progress in Fabrication. London: intechopen. p. 145-174. ISBN 978-1-83768-471-7. https://www.intechopen.com/chapters/85515.
- ↑ Karp, Gerald; van der Geer, Peter (2004). Cell and molecular biology: concepts and experiments (4th ed., Wiley International ed.). New York: J. Wiley & Sons. p. 223. ISBN 978-0471656654.
Further reading
- "Interpretation of the atmospheric oxygen bands; electronic levels of the oxygen molecule". Nature 122 (3075): 505. 1928. doi:10.1038/122505a0. Bibcode: 1928Natur.122..505M.
- Chou, Pi-Tai; Wei, Guor-Tzo; Lin, Chih-Hung; Wei, Ching-Yen; Chang, Chie-Hung (1996-01-01). "Direct Spectroscopic Evidence of Photosensitized O2 765 nm (1Σ+g → 3Σ-g) and O2 Dimol 634 and 703 nm ((1Δg)2 → (3Σ-g)2) Vibronic Emission in Solution". Journal of the American Chemical Society 118 (12): 3031–3032. doi:10.1021/ja952352p. ISSN 0002-7863.
- Földes, T.; Čermák, P.; Macko, M.; Veis, P.; Macko, P. (January 2009). "Cavity ring-down spectroscopy of singlet oxygen generated in microwave plasma". Chemical Physics Letters 467 (4–6): 233–236. doi:10.1016/j.cplett.2008.11.040. Bibcode: 2009CPL...467..233F.
- "Singlet oxygen formation in photocatalytic TiO₂ aqueous suspension". Phys. Chem. Chem. Phys. 6 (11): 2917–2918. 2004. doi:10.1039/B405084C. Bibcode: 2004PCCP....6.2917N.
- Bodner, G.M. (2002) Lecture Demonstration Movie Sheets: 8.4 Liquid Oxygen—Paramagnetism and Color, West Lafayette, IN, USA: Purdue University Department of Chemistry, see Liquid Oxygen---Paramagnetism and Color and Lecture Demonstration Movie Sheets, accessed 11 August 2015; alternatively, see Bodner, G.M.; K. Keyes & T.J. Greenbowe (1995) Purdue University Lecture Demonstration Manual, 2nd Edn, p. TBD, New York, NY, USA: John Wiley and Sons. [Earlier appearing reference on magnetic properties of oxygen states.]
External links
- The NIST webbook on oxygen
- Photochemistry & Photobiology tutorial on Singlet Oxygen
- Demonstration of the Red Singlet Oxygen Dimol Emission (Purdue University)
 |

