Chemistry:Otenaproxesul
 | |
 | |
| Clinical data | |
|---|---|
| Other names | ATB-352[1] |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
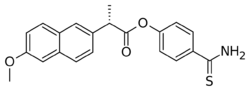
| Formula | C21H19NO3S |
| Molar mass | 365.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Otenaproxesul is a analgesic and anti-inflammatory drug being developed by Antibe Therapeutics. An NSAID structurally derived from naproxen, in 2016 it received approval to commence phase II clinical trials as a treatment for osteoarthritis after completing phase I clinical trials in 2015.[2] In 2018, the drug completed trials for gastrointestinal safety, and in 2020 completed phase IIb trials on efficacy of pain reduction.[3] Initial phase III clinical trials in 2021 failed to meet the necessary criteria to advance to the next phase.
Other in vivo studies have demonstrated a reduction in zymosan-induced pain and inflammation and cytokine-induced bone loss.[4] Preclinical studies have also investigated the treatment of melanoma, intestinal cancer,[5] and periodontitis.[6]
Pharmacology
Like other NSAIDs, otenaproxesul acts as an inhibitor of the cycloxygenase (COX) enzymes, suppressing the production of prostaglandins. Additionally, it releases hydrogen sulfide in the gastrointestinal tract, reducing gastrointestinal adverse effects such as ulcers.[7]
References
- ↑ "4-Carbamothioylphenyl 2-(6-methoxynaphthalen-2-yl)propanoate". National Institute of Health. https://pubchem.ncbi.nlm.nih.gov/compound/25065981.
- ↑ "Otenaproxesul". January 27, 2021. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=summary&ligandId=9534.
- ↑ "Otenaproxesul (ATB-346)". 19 July 2019. http://antibethera.com/pipeline/atb-346/.
- ↑ "Otenaproxesul, (+/-)-". https://drugs.ncats.io/drug/3096O7WP53.
- ↑ "Enhanced chemopreventive effects of a hydrogen sulfide-releasing anti-inflammatory drug (ATB-346) in experimental colorectal cancer.". Nitric Oxide 41: 131–7. September 15, 2014. doi:10.1016/j.niox.2014.04.006. PMID 24747869. https://pubmed.ncbi.nlm.nih.gov/24747869/.
- ↑ "Anti-inflammatory effect of ATB-352, a H2S -releasing ketoprofen derivative, on lipopolysaccharide-induced periodontitis in rats". Pharmacological Research 132: 220–231. June 2018. doi:10.1016/j.phrs.2017.12.022. PMID 29287688.
- ↑ "Enhanced Analgesic Effects and Gastrointestinal Safety of a Novel, Hydrogen Sulfide-Releasing Anti-Inflammatory Drug (ATB-352): A Role for Endogenous Cannabinoids". Antioxidants & Redox Signaling 33 (14): 1003–1009. November 2020. doi:10.1089/ars.2019.7884. PMID 32064887.
 |

