Chemistry:Palmatine
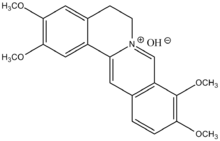
| |
| Names | |
|---|---|
| IUPAC name
2,3,9,10-tetramethoxy-5,6-dihydroisoquinolino[2,1-b]isoquinolin-7-ium
| |
| Other names
Berbericinine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C21H22NO4+ | |
| Molar mass | 352.4083 g/mol |
| Density | 1.23 g/cm3 |
| Boiling point | 482.9 °C (901.2 °F; 756.0 K) at 760 mmHg |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Palmatine is a protoberberine alkaloid found in several plants including Phellodendron amurense, Coptis Chinensis[1] (Rhizoma coptidis, chinese goldthread) and Corydalis yanhusuo,[2] Tinospora cordifolia[3] (gurjo, heart-leaved moonseed), Tinospora sagittata,[4] Phellodendron amurense[5] (amur cork tree), Stephania yunnanensis.[6]
It is the major component of the protoberberine extract from Enantia chlorantha.[7]
It has been studied for its potential use in the treatment of jaundice, dysentery, hypertension, inflammation, and liver-related diseases.[8] This compound also has weak in vitro activity against flavivirus.[9]
Pharmacology
Neuroprotective activity
Palmatine can be used to treat Alzheimer’s disease, mainly by inhibiting the activity of acetylcholinesterase (AChE), butyrylcholinesterase (BChE) and neuraminidase-1 (NA-1). It was found, that the positively charged nitrogen on palmatine binds in the gorge of active sire of AChE.[10]
Research show that palmatine had antidepressant effect. It was achieved by regulating brain catalase levels, monoamine oxidase-A (MAO-A) activity, lipid peroxidation, plasma nitrite and corticosterone levels.[11]
Regulating blood lipid activity
Palmatine achieved hypoglycemic effects by inducing insulin release and insulin-mimicking activity.[12][13] In addition, studies found that palmatine also inhibited the activity of lens aldose reductase,[14] sucrase and maltase.[15] In vivo research showed that palmatine reduced serum total cholesterol (TC) and triglycerides (TG) and increased serum high-density lipoprotein cholesterol.[16]
Anticancer activity
Research showed that palmatine had broad anti-cancer activity. Palmatine had significant growth inhibitory effects on seven human cancer cell lines: 7701QGY, SMMC7721, HepG2, CEM, CEM/VCR, K III and Lewis.[17] In addition, palmatine also had anti-cancer activity on MCF-7, U251, KB,[18] CHOK-1, HT-29 and SiHacell lines.[19] Palmatine induced apoptosis in human skin epithelial carcinoma cells (A431) in a concentration- and time-dependent manner via damaging severely to DNA and inhibiting the activity of Bcl-2 protein.[20][21][22] In addition, palmatine can inhibit the proliferation and infiltration of cancer cells.
Antibacterial and antiviral activity
Palmitine has inhibitory effect on Gram-positive bacteria which is significantly stronger than that on Gram-negative bacteria,[23] and 9-O-substituted palmatine derivatives exhibited stronger antibacterial activity.[24][25]
Anti-inflammatory activity
Study showed, that palmatine can decrease the production of pro-inflammatory factors and increase the production of anti-inflammatory factors.[26]
Other pharmacological activity
Studies showed that palmatine had antioxidant activity,[27][28] had a protective effect on gastric ulcer,[29] derivatives of palmatine were more effective against ulcerative colitis, including low cytotoxicity tointestinal epithelial cells.[30] In addition, palmatine might have the antiarrhythmic effect,[31] and provideprotection from myocardial ischemia-reperfusion injury.[32]
Toxicity
A large number of studies have shown that palmatine has a complex effect on the metabolism of enzymes in the liver, and that palmatine has significant DNA toxicity.[33] However, some 9-O-substituted palmatine derivatives exhibited less toxic than palmatine.[34] In addition, palmatine had higher affinity to nucleic acids than serum proteins, which make them suitable candidates for delivery by serum proteins.[35]
See also
References
- ↑ Yang, Seong Baek; Kim, Eun Hee; Kim, Seung Hee; Kim, Young Hun; Oh, Weontae; Lee, Jin-Tae; Jang, Young-Ah; Sabina, Yeasmin et al. (2018-09-17). "Electrospinning Fabrication of Poly(vinyl alcohol)/Coptis chinensis Extract Nanofibers for Antimicrobial Exploits". Nanomaterials 8 (9): 734. doi:10.3390/nano8090734. ISSN 2079-4991. PMID 30227671.
- ↑ Zhang, Qian; Chen, Cen; Wang, Feng-Qin; Li, Chun-Hong; Zhang, Qi-Hui; Hu, Yuan-Jia; Xia, Zhi-Ning; Yang, Feng-Qing (2016-12-01). "Simultaneous screening and analysis of antiplatelet aggregation active alkaloids from Rhizoma Corydalis". Pharmaceutical Biology 54 (12): 3113–3120. doi:10.1080/13880209.2016.1211714. ISSN 1388-0209. PMID 27558975.
- ↑ Kumar, Peeyush; Srivastava, Vartika; Chaturvedi, Rakhi; Sundar, Durai; Bisaria, V. S. (2017-08-01). "Elicitor enhanced production of protoberberine alkaloids from in vitro cell suspension cultures of Tinospora cordifolia (Willd.) Miers ex Hook. F. & Thoms" (in en). Plant Cell, Tissue and Organ Culture 130 (2): 417–426. doi:10.1007/s11240-017-1237-0. ISSN 1573-5044. https://doi.org/10.1007/s11240-017-1237-0.
- ↑ Rong, Qian; Xu, Min; Dong, Qi; Zhang, Yuli; Li, Yinglun; Ye, Gang; Zhao, Ling (2016-08-30). "In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori". BMC Complementary and Alternative Medicine 16 (1): 331. doi:10.1186/s12906-016-1310-y. ISSN 1472-6882. PMID 27576439.
- ↑ Sun, Minglong; Xu, Lijiao; Peng, Yingli; Liu, Tong; Zhang, Yuhong; Zhou, Zhiqiang (2016-04-01). "Multiscale analysis of the contents of palmatine in the Nature populations of Phellodendron amurense in Northeast China" (in en). Journal of Forestry Research 27 (2): 265–272. doi:10.1007/s11676-015-0200-3. ISSN 1993-0607. https://doi.org/10.1007/s11676-015-0200-3.
- ↑ Xin, Aiyi; Zhang, Yaming; Zhang, Yanxia; Di, Duolong; Liu, Junxi (October 2018). "Development of an HPLC-DAD method for the determination of five alkaloids in Stephania yunnanensis Lo and in rat plasma after oral dose of Stephania yunnanensis Lo extracts". Biomedical Chromatography 32 (10): e4292. doi:10.1002/bmc.4292. ISSN 1099-0801. PMID 29782649.
- ↑ Virtanen P., Njimi T., Ekotto Mengata D.: Clinical trials of hepatitis cure with protoberberine alkaloids of Enantia Chlorantha (abstract) Eur.J.Clin.Pharmacol.36: A123, 1989b
- ↑ "Therapeutic potential of nucleic acid-binding isoquinoline alkaloids: Binding aspects and implications for drug design". Medicinal Research Reviews 31 (6): 821–862. January 2010. doi:10.1002/med.20202. PMID 20077560.
- ↑ Jia F., Zou G., Fan J., Yuan Z."Identification of palmatine as an inhibitor of West Nile virus" Archives of Virology 2010 155:8 (1325-1329)
- ↑ Mukherjee, Pulok K.; Kumar, Venkatesan; Mal, Mainak; Houghton, Peter J. (2007-04-10). "Acetylcholinesterase inhibitors from plants" (in en). Phytomedicine 14 (4): 289–300. doi:10.1016/j.phymed.2007.02.002. ISSN 0944-7113. PMID 17346955. https://www.sciencedirect.com/science/article/abs/pii/S094471130700030X.
- ↑ Dhingra, Dinesh; Bhankher, Arun (2014-02-01). "Behavioral and biochemical evidences for antidepressant-like activity of palmatine in mice subjected to chronic unpredictable mild stress" (in en). Pharmacological Reports 66 (1): 1–9. doi:10.1016/j.pharep.2013.06.001. ISSN 1734-1140. PMID 24905299. https://www.sciencedirect.com/science/article/abs/pii/S1734114014000024.
- ↑ Patel, Mayurkumar B.; Mishra, Shrihari (September 2011). "Hypoglycemic activity of alkaloidal fraction of Tinospora cordifolia". Phytomedicine 18 (12): 1045–1052. doi:10.1016/j.phymed.2011.05.006. ISSN 0944-7113. PMID 21665451. http://dx.doi.org/10.1016/j.phymed.2011.05.006.
- ↑ Ma, Bingxin; Tong, Jing; Zhou, Gao; Mo, Qigui; He, Jingsheng; Wang, Youwei (March 2016). "Coptis chinensis inflorescence ameliorates hyperglycaemia in 3T3-L1 preadipocyte and streptozotocin-induced diabetic mice". Journal of Functional Foods 21: 455–462. doi:10.1016/j.jff.2015.12.021. ISSN 1756-4646. http://dx.doi.org/10.1016/j.jff.2015.12.021.
- ↑ Patel, Mayurkumar B.; Mishra, Shrihari (2012-02-01). "Isoquinoline Alkaloids from Tinospora cordifolia Inhibit Rat Lens Aldose Reductase". Phytotherapy Research 26 (9): 1342–1347. doi:10.1002/ptr.3721. ISSN 0951-418X. PMID 22294283. http://dx.doi.org/10.1002/ptr.3721.
- ↑ Patel, Mayurkumar B.; Mishra, Shrihari M. (January 2012). "Magnoflorine from Tinospora cordifolia stem inhibits α-glucosidase and is antiglycemic in rats". Journal of Functional Foods 4 (1): 79–86. doi:10.1016/j.jff.2011.08.002. ISSN 1756-4646.
- ↑ Ma, Hang; Hu, Yinran; Zou, Zongyao; Feng, Min; Ye, Xiaoli; Li, Xuegang (2016-04-04). "Antihyperglycemia and Antihyperlipidemia Effect of Protoberberine Alkaloids From Rhizoma Coptidis in HepG2 Cell and Diabetic KK-Ay Mice". Drug Development Research 77 (4): 163–170. doi:10.1002/ddr.21302. ISSN 0272-4391. PMID 27045983. http://dx.doi.org/10.1002/ddr.21302.
- ↑ Zhang, Lei; Li, Jingjing; Ma, Fei; Yao, Shining; Li, Naisan; Wang, Jing; Wang, Yongbin; Wang, Xiuzhen et al. (2012-09-25). "Synthesis and Cytotoxicity Evaluation of 13-n-Alkyl Berberine and Palmatine Analogues as Anticancer Agents". Molecules 17 (10): 11294–11302. doi:10.3390/molecules171011294. ISSN 1420-3049. PMID 23011273.
- ↑ Costa, Emmanoel Vilaça; Cruz, Pedro Ernesto O. da; Pinheiro, Maria Lúcia B.; Marques, Francisco A.; Ruiz, Ana Lúcia T. G.; Marchetti, Gabriela M.; Carvalho, João Ernesto de; Barison, Andersson et al. (2013). "Aporphine and Tetrahydroprotoberberine Alkaloids from the Leaves ofGuatteria friesiana(Annonaceae) and their Cytotoxic Activities". Journal of the Brazilian Chemical Society. doi:10.5935/0103-5053.20130103. ISSN 0103-5053.
- ↑ Bala, Manju; Pratap, Kunal; Verma, Praveen Kumar; Singh, Bikram; Padwad, Yogendra (December 2015). "Validation of ethnomedicinal potential of Tinospora cordifolia for anticancer and immunomodulatory activities and quantification of bioactive molecules by HPTLC". Journal of Ethnopharmacology 175: 131–137. doi:10.1016/j.jep.2015.08.001. ISSN 0378-8741. PMID 26253577. http://dx.doi.org/10.1016/j.jep.2015.08.001.
- ↑ Ali, Daoud; Ali, Huma (2014-07-03). "Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells". Toxicological & Environmental Chemistry 96 (6): 941–950. doi:10.1080/02772248.2014.987510. ISSN 0277-2248. http://dx.doi.org/10.1080/02772248.2014.987510.
- ↑ Wu, Juan; Xiao, Qicai; Zhang, Na; Xue, Changhu; Leung, Albert Wingnang; Zhang, Hongwei; Tang, Qing-Juan; Xu, Chuanshan (September 2016). "Palmatine hydrochloride mediated photodynamic inactivation of breast cancer MCF-7 cells: Effectiveness and mechanism of action". Photodiagnosis and Photodynamic Therapy 15: 133–138. doi:10.1016/j.pdpdt.2016.07.006. ISSN 1572-1000. PMID 27444887. http://dx.doi.org/10.1016/j.pdpdt.2016.07.006.
- ↑ He, Qiyuan; Zhang, Hua (2020). "Towards the Scalable vdW Heterostructure Array". Acta Physico-Chimica Sinica: 2003075–0. doi:10.3866/pku.whxb202003075. ISSN 1000-6818.
- ↑ Deng, Yecheng; Zhang, Ming; Luo, Haiyu (May 2012). "Identification and antimicrobial activity of two alkaloids from traditional Chinese medicinal plant Tinospora capillipes". Industrial Crops and Products 37 (1): 298–302. doi:10.1016/j.indcrop.2011.12.006. ISSN 0926-6690. http://dx.doi.org/10.1016/j.indcrop.2011.12.006.
- ↑ Li, ZC; Kong, XB; Mai, WP; Sun, GC; Zhao, SZ (2015). "Synthesis and antimicrobial activity of 9-o-substituted palmatine derivatives". Indian Journal of Pharmaceutical Sciences 77 (2): 196–201. doi:10.4103/0250-474x.156588. ISSN 0250-474X. PMID 26009653.
- ↑ Song, Li; Zhang, Hai-Jing; Deng, An-Jun; Li, Jia; Li, Xiang; Li, Zhi-Hong; Zhang, Zhi-Hui; Wu, Lian-Qiu et al. (May 2018). "Syntheses and structure-activity relationships on antibacterial and anti-ulcerative colitis properties of quaternary 13-substituted palmatines and 8-oxo-13-substituted dihydropalmatines". Bioorganic & Medicinal Chemistry 26 (9): 2586–2598. doi:10.1016/j.bmc.2018.04.025. ISSN 0968-0896. PMID 29680749. http://dx.doi.org/10.1016/j.bmc.2018.04.025.
- ↑ Yan, Baoqi; Wang, Dongsheng; Dong, Shuwei; Cheng, Zhangrui; Na, Lidong; Sang, Mengqi; Yang, Hongzao; Yang, Zhiqiang et al. (April 2017). "Palmatine inhibits TRIF-dependent NF-κB pathway against inflammation induced by LPS in goat endometrial epithelial cells". International Immunopharmacology 45: 194–200. doi:10.1016/j.intimp.2017.02.004. ISSN 1567-5769. PMID 28236763. http://dx.doi.org/10.1016/j.intimp.2017.02.004.
- ↑ Ma, Bingxin; Zhu, Ling; Zang, Xiaoyan; Chen, Yuxin; Li, Dong; Wang, Youwei (October 2013). "Coptis chinensis inflorescence and its main alkaloids protect against ultraviolet-B-induced oxidative damage". Journal of Functional Foods 5 (4): 1665–1672. doi:10.1016/j.jff.2013.07.010. ISSN 1756-4646. http://dx.doi.org/10.1016/j.jff.2013.07.010.
- ↑ Shia, Chi-Sheng; Hou, Yu-Chi; Juang, Shin-Hun; Tsai, Shang-Yuan; Hsieh, Pei-Hsun; Ho, Lu-Ching; Chao, Pei-Dawn Lee (2011). "Metabolism and Pharmacokinetics of San-Huang-Xie-Xin-Tang, a Polyphenol-Rich Chinese Medicine Formula, in Rats andEx-VivoAntioxidant Activity". Evidence-Based Complementary and Alternative Medicine 2011: 721293. doi:10.1093/ecam/nep124. ISSN 1741-427X. PMID 19737807.
- ↑ Wang, Wei-Min; Nwabueze, OkechukwuPatrick; Yang, Hui; Zhai, Hong-Bin (2018). "High-performance liquid chromatography identification of gastroprotective and antioxidant effects of purified fractions A-E from the stem of Coscinium fenestratum". Pharmacognosy Magazine 14 (55): 78. doi:10.4103/pm.pm_267_17. ISSN 0973-1296.
- ↑ Song, Li; Zhang, Hai-Jing; Deng, An-Jun; Li, Jia; Li, Xiang; Li, Zhi-Hong; Zhang, Zhi-Hui; Wu, Lian-Qiu et al. (May 2018). "Syntheses and structure-activity relationships on antibacterial and anti-ulcerative colitis properties of quaternary 13-substituted palmatines and 8-oxo-13-substituted dihydropalmatines". Bioorganic & Medicinal Chemistry 26 (9): 2586–2598. doi:10.1016/j.bmc.2018.04.025. ISSN 0968-0896. PMID 29680749. http://dx.doi.org/10.1016/j.bmc.2018.04.025.
- ↑ Guo, Rui; Zhang, Xiaoxiao; Su, Jin; Xu, Haiyu; Zhang, Yanqiong; Zhang, Fangbo; Li, Defeng; Zhang, Yi et al. (May 2018). "Identifying potential quality markers of Xin-Su-Ning capsules acting on arrhythmia by integrating UHPLC-LTQ-Orbitrap, ADME prediction and network target analysis". Phytomedicine 44: 117–128. doi:10.1016/j.phymed.2018.01.019. ISSN 0944-7113. PMID 29526583. http://dx.doi.org/10.1016/j.phymed.2018.01.019.
- ↑ Tan, Hui-Li; Chan, Kok-Gan; Pusparajah, Priyia; Duangjai, Acharaporn; Saokaew, Surasak; Mehmood Khan, Tahir; Lee, Learn-Han; Goh, Bey-Hing (2016-10-07). "Rhizoma Coptidis: A Potential Cardiovascular Protective Agent". Frontiers in Pharmacology 7: 362. doi:10.3389/fphar.2016.00362. ISSN 1663-9812. PMID 27774066.
- ↑ Ali, Daoud; Ali, Huma (2014-07-03). "Assessment of DNA damage and cytotoxicity of palmatine on human skin epithelial carcinoma cells". Toxicological & Environmental Chemistry 96 (6): 941–950. doi:10.1080/02772248.2014.987510. ISSN 0277-2248. http://dx.doi.org/10.1080/02772248.2014.987510.
- ↑ Li, ZC; Kong, XB; Mai, WP; Sun, GC; Zhao, SZ (2015). "Synthesis and antimicrobial activity of 9-o-substituted palmatine derivatives". Indian Journal of Pharmaceutical Sciences 77 (2): 196–201. doi:10.4103/0250-474x.156588. ISSN 0250-474X. PMID 26009653.
- ↑ Khan, Asma Yasmeen; Suresh Kumar, Gopinatha (2015-12-01). "Natural isoquinoline alkaloids: binding aspects to functional proteins, serum albumins, hemoglobin, and lysozyme" (in en). Biophysical Reviews 7 (4): 407–420. doi:10.1007/s12551-015-0183-5. ISSN 1867-2469. PMID 28510102.
 |

