Chemistry:Sinapine
| |
| Names | |
|---|---|
| Preferred IUPAC name
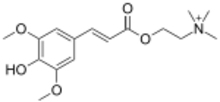
2-{[(2E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)prop-2-enoyl]oxy}-N,N,N-trimethylethan-1-aminium | |
| Other names
Sinapoylcholine; Sinapic acid choline ester
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C16H24NO5 | |
| Molar mass | 310.370 g·mol−1 |
| Melting point | 178 °C (352 °F; 451 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sinapine is an alkaloidal amine found in some seeds, particularly oil seeds of plants in the family Brassicaceae.[2] It is the choline ester of sinapic acid.
Sinapine was discovered by Etienne Ossian Henry in 1825.[3]
Occurrence
Sinapine typically occurs in the outer seed coat of oil crops and is plentiful in some types of press cake leftover after vegetable oil extraction.[2] Typical oil seed cake residues high in sinapine include Brassica juncea (1.22% by mass),[4] and rapeseed (0.39-1.06% by mass).[5]
Isolation
The typical protocol for extracting Sinapine from seed cakes entails defatting the cake with hexane via a Soxhlet apparatus followed by extraction with 70% methanol held at 75 °C.[6]
Metabolism
Sinapine esterase is an enzyme whose two substrates are sinapine and H2O and whose two products are sinapic acid and choline.
Sinapoylglucose—choline O-sinapoyltransferase is an enzyme whose two substrates are 1-O-sinapoyl-β-D-glucose and choline, whereas its two products are D-glucose and sinapine.
See also
- Phenolic content in wine
- Syringaldehyde
- Syringol
- Syringic acid
- Acetosyringone
- Sinapyl alcohol
- Sinapinic acid
- Sinapaldehyde
- Canolol
References
- ↑ Gmelin, R; Bredenberg JB, son (February 1966). "[Studies on the constituents of various Erysimum varieties: a) identification of the bitter substance erysimupicrone as strophanthidin; b) glucosinolates in the seeds of Erysimum perofskianum Fisch et Mey., E. Allionii hort., E. crepidifolium Rohb. and E. cheiranthoides L]." (in German). Arzneimittel-Forschung 16 (2): 123–7. PMID 6014002.
- ↑ 2.0 2.1 Niciforovic, Neda; Abramovi, Helena (2014). "Sinapic Acid and Its Derivatives: Natural Sources and Bioactivity". Comprehensive Reviews in Food Science and Food Safety 13 (1): 34–51. doi:10.1111/1541-4337.12041. PMID 33412688.
- ↑ Tzagoloff, A. (1963). "Metabolism of Sinapine in Mustard Plants. I. Degradation of Sinapine into Sinapic Acid & Choline". Plant Physiology 38 (2): 202–206. doi:10.1104/pp.38.2.202. PMID 16655775.
- ↑ Matthäus, B .; Zubr, J. (2000). "Variability of specific components in Camelina sativa oilseed cakes". Industrial Crops and Products 12 (1): 9–18. doi:10.1016/S0926-6690(99)00040-0.
- ↑ Vuorela, Satu (2005). Analysis, isolation, and bioactivities of rapeseed phenolics. Helsinki, Finland: University of Helsinki. pp. 19–20. ISBN 9789521027215. https://helda.helsinki.fi/bitstream/handle/10138/20877/analysis.pdf?sequence=1. Retrieved 14 June 2014.
- ↑ Vuorela, Satu (2005). Analysis, isolation, and bioactivities of rapeseed phenolics. Helsinki, Finland: University of Helsinki. pp. 19–20. ISBN 9789521027215. https://helda.helsinki.fi/bitstream/handle/10138/20877/analysis.pdf?sequence=1. Retrieved 14 June 2014.
 |

