Chemistry:Trimethylsulfoxonium
From HandWiki
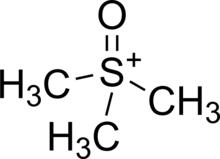
| |
| Names | |
|---|---|
| IUPAC name
Trimethyl(oxo)-λ4-sulfanium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C3H9OS+ | |
| Molar mass | 93.16 g·mol−1 |
| Related compounds | |
Related compounds
|
trimethylsulfonium; trimethylselenoxonium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trimethylsulfoxonium (abbreviated TMSO) is a cation with a formula (CH3)3SO+ consisting of a sulfur atom attached to three methyl groups and one oxygen atom. It has a net charge of +1.
Production
Refluxing dimethyl sulfoxide with methyl iodide can yield trimethylsulfoxonium iodide.[1]
Reactions
Treated with sodium hydride, trimethylsulfoxonium forms dimethylsulfoxonium methylide.[1]
Trimethylsulfoxonium can polymerise to yield polyethylene.[2][3]
Copper, zinc and palladium ions in water react with trimethylsulfoxonium and sodium hydroxide to form sulfur ylide complexes.[4]
Properties
In the chloride, the sulfur-oxygen bond length is 1.436 Å, sulfur-carbon bond is 1.742. OSC angles are 112.6°, and CSC angles are 106.2°.[5]
List of compounds
| name | formula | crystal Å | volume | density | comment | reference |
|---|---|---|---|---|---|---|
| trimethylsulfoxonium phthalimide | [C3H9SO][C8H4NO2] | orthorhombic Pnma a=8.445 b=10.958 c=12.084 Z=4 | 1118.2 | colourless | [6] | |
| Trimethylsulfoxonium nitrate | [7] | |||||
| Trimethylsulfoxonium tetraphenylborate | [(CH3)3SO]Ph4B | P21/m a=9.482 b=12.790 c=9.713 β=106.48° Z=2 | 1130 | 1.177 | [5] | |
| Trimethylsulfoxonium chloride | [(CH3)3SO]Cl | white; sublime 227°C | [7][1] | |||
| Trimethylsulfoxonium chromate | [7] | |||||
| trimethylsulfoxonium tetrachloridocobaltate(II) | [(CH3)3SO]2CoCl4 | monoclinic, P21/c a = 8.4397, b = 15.6692, c = 12.5989, β = 93.858°, Z = 4 | 1662.3 | blue | [8] | |
| Trimethylsulfoxonium bromide | [7] | |||||
| Trimethylsulfoxonium iodide | [(CH3)3SO]I | yellow; dec 170°C | [7][1] | |||
| [(CH3)3SO]Cu2I3 | Pnma | [9] | ||||
| [(CH3)3SO]Ag2I3 | Pnma | [9] | ||||
| trimethyloxosulfonium trichloro-cadmate | [(CH3)3SO]CdCl3 | orthorhombic Pnma a=6.688 b=10.147 c=13.446 Z=4 | 912.5 | 2.270 | [7] | |
| trimethyloxosulfonium tribromo-cadmate | [(CH3)3SO]CdBr3 | orthorhombic Pnma a=6.946 b=10.543 c=13.782 Z=4 | 1009.3 | 2.928 | colourless | [7] |
| bis(trimethylsulfoxonium) catena-poly[µ2-hexabromido-indium(III)sodium(I)] | [(CH3)3SO]2NaInBr6 | orthorhombic, Pmn21, a = 10.5451, b = 7.1169, c = 13.7034, Z = 2 | 1028.42 | colourless | [10] | |
| [(CH3)3SO]PbBr3 | orthorhombic Pnma a=7.5697 b=10.7768 c=13.9143 Z=4 | 1135.09 | 3.160 | white | [11] | |
| [(CH3)3SO]PbI3 | orthorhombic Pnma a=7.8157 b=11.1996 c=14.3780 Z=4 | 1260.29 | 3.590 | light yellow; band gap 2.30 eV | [12] | |
| [(CH3)3SO]3Bi2Br9 | orthorhombic Pnma a=7.0351 b=10.6613 c=13.9190 | 1043.98 | 3.004 | light yellow | [11] | |
| [(CH3)3SO]3Bi2I9 | orthorhombic Pnma a=7.4530 b=11.2169 c=14.4009 | 1203.92 | 3.383 | red | [11] |
References
- ↑ 1.0 1.1 1.2 1.3 Ng, John S.; Liu, Chin; McGarrigle, Eoghan M.; Aggarwal, Varinder K. (2007-03-15), John Wiley & Sons, Ltd., ed. (in en), Trimethylsulfoxonium Iodide, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/9780470842898.rt351.pub2, ISBN 978-0-471-93623-7, https://onlinelibrary.wiley.com/doi/10.1002/9780470842898.rt351.pub2, retrieved 2024-01-22
- ↑ "Polyethylene from Trimethylsulfoxonium Halide in Water". Synfacts 8 (08): 0845. August 2012. doi:10.1055/s-0032-1316727.
- ↑ Luo, Jun; Lu, Fangfang; Shea, Kenneth J. (15 May 2012). "Hydrocarbon Waxes from a Salt in Water: The C1 Polymerization of Trimethylsulfoxonium Halide". ACS Macro Letters 1 (5): 560–563. doi:10.1021/mz300140x. PMID 35607061.
- ↑ Lin, Ivan J.B.; Hwan, Luchen; Shy, Hwai C.; Chen, M.C.; Wang, Y. (November 1986). "The synthesis of sulfur ylide complexes of palladium, zinc and copper in water". Journal of Organometallic Chemistry 315 (1): 135–142. doi:10.1016/0022-328X(86)80418-1.
- ↑ 5.0 5.1 Knop, Osvald; Cameron, T. Stanley; Bakshi, Pradip K.; Linden, Antony; Roe, Stephen P. (1994-08-01). "Crystal chemistry of tetraradial species. Part 5. Interaction between cation lone pairs and phenyl groups in tetraphenylborates: crystal structures of Me 3 S + ,Et 3 S + ,Me 3 SO + ,Ph 2 I + , and 1-azoniapropellane tetraphenylborates" (in en). Canadian Journal of Chemistry 72 (8): 1870–1881. doi:10.1139/v94-238. ISSN 0008-4042. http://www.nrcresearchpress.com/doi/10.1139/v94-238.
- ↑ Mallah, Eyad; Abu-Salem, Qutaiba; Dayyih, Wael Abu; Hamad, Mohammed; Steimann, Manfred; Maichle-Mössmer, Caecilia (2011-01-01). "Crystal structure of trimethylsulfoxonium phthalimide, [C3H9SO[C8H4NO2]"]. Zeitschrift für Kristallographie - New Crystal Structures 226 (3). doi:10.1524/ncrs.2011.0155. ISSN 2197-4578. https://www.degruyter.com/document/doi/10.1524/ncrs.2011.0155/html.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Puget, R.; Jannin, M.; de Brauer, C.; Perret, R. (1991-09-15). "Structures oftrimethyloxosulfonium salts. V. The catena-tri-μ-chloro-cadmate and the catena-tri-μ-bromo-cadmate". Acta Crystallographica Section C Crystal Structure Communications 47 (9): 1803–1805. doi:10.1107/S0108270190013701. Bibcode: 1991AcCrC..47.1803P. https://scripts.iucr.org/cgi-bin/paper?S0108270190013701.
- ↑ Zhong, Xin; Zhou, Junyan; Hao, Munan (2020-06-25). "Crystal structure of trimethylsulfoxonium tetrachloridocobaltate(II) [(CH 3 ) 3 SO 2 CoCl 4"] (in en). Zeitschrift für Kristallographie - New Crystal Structures 235 (4): 1001–1002. doi:10.1515/ncrs-2020-0159. ISSN 2197-4578. https://www.degruyter.com/document/doi/10.1515/ncrs-2020-0159/html.
- ↑ 9.0 9.1 Pinky, Tamanna; Popy, Dilruba A.; Zhang, Zheng; Jiang, Jie; Pachter, Ruth; Saparov, Bayram (18 January 2024). "Synthesis and Characterization of New Hybrid Organic–Inorganic Metal Halides [(CH 3 ) 3 SO]M 2 I 3 (M = Cu and Ag)". Inorganic Chemistry. doi:10.1021/acs.inorgchem.3c04119.
- ↑ Jin, Shifeng; Lu, Jiali (2023-12-15). "The crystal structure of bis(trimethylsulfoxonium) catena -poly[µ 2 -hexabromido-indium(III)sodium(I) C 6 H 18 O 2 S 2 NaInBr 6"] (in en). Zeitschrift für Kristallographie - New Crystal Structures 238 (6): 1171–1173. doi:10.1515/ncrs-2023-0377. ISSN 1433-7266. https://www.degruyter.com/document/doi/10.1515/ncrs-2023-0377/html.
- ↑ 11.0 11.1 11.2 Pipitone, Candida; Giannici, Francesco; Martorana, Antonino; García-Espejo, Gonzalo; Carlotto, Silvia; Casarin, Maurizio; Guagliardi, Antonietta; Masciocchi, Norberto (2021-06-03). "Heterovalent Bi III /Pb II Ionic Substitution in One-Dimensional Trimethylsulfoxonium Halide Pseudo-Perovskites (X = I, Br)" (in en). The Journal of Physical Chemistry C 125 (21): 11728–11742. doi:10.1021/acs.jpcc.1c02571. ISSN 1932-7447. https://pubs.acs.org/doi/10.1021/acs.jpcc.1c02571.
- ↑ Rahman, Md. Mahbubur; Ge, Chuang-ye; Yoo, Kicheon; Lee, Jae-Joon (September 2021). "Aqueous phase synthesis of trimethylsulfoxonium lead triiodide for moisture-stable perovskite solar cells". Materials Today Energy 21: 100803. doi:10.1016/j.mtener.2021.100803.
 |

