Chemistry:Trimethylsulfonium
| |
| Names | |
|---|---|
| IUPAC name
Trimethylsulfonium
| |
| Systematic IUPAC name
Trimethylsulfanium | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
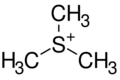
| (CH 3) 3S+ | |
| Molar mass | 77.17 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
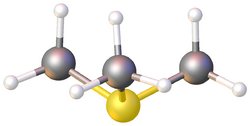
Trimethylsulfonium (systematically named trimethylsulfanium) is an organic cation with the chemical formula (CH
3)
3S+
(also written as C
3H
9S+
).
Compounds
Several salts of trimethylsulfonium are known. X-ray crystallography reveals that the ion has trigonal pyramidal molecular geometry at sulfur atom, with C-S-C angles near 102° and C-S bond distance of 177 picometers. Unless the counteranion is colored, all trimethylsulfonium salts are white or colorless.
| Salt | Formula | Molecular weight (g/mol) | Properties[2] |
|---|---|---|---|
| Trimethylsulfonium chloride | [(CH 3) 3S]+ Cl− |
112.5 | Colorless crystals, decompose at 100 °C, very soluble in ethanol, very hygroscopic.[3] |
| Trimethylsulfonium bromide | [(CH 3) 3S]+ Br− |
157 | Colorless crystals. Decomposes at 172 °C, melts in a sealed tube at 201-201 °C, reacts in neutral aqueous solution.[clarification needed][4] |
| Trimethylsulfonium iodide | [(CH 3) 3S]+ I− |
204 | Colorless crystals, decomposes at 203-207 °C.[4][5] crystal structure monoclinic, with these parameters: a = 5.94 μm, b = 8.00 μm, c = 8.92 μm, β = 126°32′ 2 formulas per unit cell,[clarification needed] density = 1.958 g/cm3[6] |
| Trimethylsulfonium tetrafluoroborate | [(CH 3) 3S]+ [BF 4]− |
163.97 | melting point = 205-210 °C[7] |
| Trimethylsulfonium methylsulfate | [(CH 3) 3S]+ CH 3OSO− 3 |
188.27 | melting point = 92-94 °C[8] Crystal structure orthorhombic with these parameters: a = 12.6157 μm, b = 8.2419 μm, c = 7.540 μm cell volume 784.0 2 formulas per unit cell[clarification needed] |
Preparation
Sulfonium compounds can be synthesised by treating a suitable alkyl halide with a thioether. For example, the reaction of dimethyl sulfide with iodomethane yields trimethylsulfonium iodide:
- (CH
3)
2S + CH
3I → [(CH
3)
3S]+
I−
Related
An extra oxygen atom can bond to the sulfur atom to yield the trimethylsulfoxonium ion [(CH
3)
3S=O]+
, where the sulfur atom is tetravalent and tetracoordinated.
Use
Glyphosate herbicide is often supplied as a trimethylsulfonium salt, referred to as trimesium.[9]
When mixed with aluminium bromide, or aluminium chloride or even hydrogen bromide, trimethylsulfonium bromide forms an ionic liquid, which melts at temperatures below standard conditions.[10]
References
- ↑ Knop, Osvald; Cameron, T. Stanley; Bakshi, Pradip K.; Linden, Antony; Roe, Stephen P. (1994). "Crystal chemistry of tetraradial species. Part 5. Interaction Between Cation Lone Pairs and Phenyl Groups in Tetraphenylborates: Crystal Structures of Me3S+,Et3S+, Me3SO+, Ph2I+, and 1-Azoniapropellane Tetraphenylborates". Canadian Journal of Chemistry 72 (8): 1870–1881. doi:10.1139/v94-238.
- ↑ Heilbron's Dictionary of Organic Compounds, volume 4, revised edition published in 1953. Published in Great Britain
- ↑ Blättler, H. (1919). "Über Trimethylsulfoniumverbindungen". Monatshefte für Chemie und verwandte Teile anderer Wissenschaften 40 (8): 417–429. doi:10.1007/BF01559085. https://zenodo.org/record/2258764.
- ↑ 4.0 4.1 Steinkopf, W.; Müller, S. (1923). "Über die Einwirkung von Jodmethyl auf Disulfide". Chem. Ber. 56 (8): 1926–1930. doi:10.1002/cber.19230560834.
- ↑ Mussgnug, F. (1941). "Trimethylammoniumjodid und Trimethylsulfoniumjodid". Naturwissenschaften 29 (17): 256. doi:10.1007/BF01479158. Bibcode: 1941NW.....29..256M.
- ↑ Zuccaro, D. Ε.; McCullough, J. D. (1 January 1959). "The crystal structure of trimethylsulfonium iodide". Zeitschrift für Kristallographie - Crystalline Materials 112 (1–6): 401–408. doi:10.1524/zkri.1959.112.jg.401.
- ↑ "Trimethylsulfonium tetrafluoroborate". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/740489. Retrieved 23 September 2016.
- ↑ "Trimethylsulfonium methyl sulfate". Sigma-Aldrich. http://www.sigmaaldrich.com/catalog/product/aldrich/303593. Retrieved 23 September 2016.
- ↑ "Glyphosate-trimesium". https://pubchem.ncbi.nlm.nih.gov/compound/Glyphosate-trimesium.
- ↑ Ma, M.; Johnson, K.E. (April 1995). "Some physicochemical characteristics of molten salts derived from trimethylsulfonium bromide". Canadian Journal of Chemistry 73 (4): 593–598. doi:10.1139/v95-076.
See also
- Onium compounds
 |