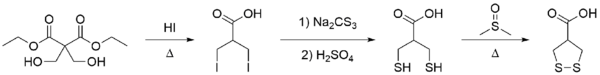Chemistry:Asparagusic acid
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Dithiolane-4-carboxylic acid | |||
| Other names
1,2-Dithiacyclopentane-4-carboxylic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C4H6O2S2 | |||
| Molar mass | 150.21 g·mol−1 | ||
| Appearance | Colorless solid | ||
| Density | 1.50 g cm−3 | ||
| Melting point | 75.7 to 76.5 °C (168.3 to 169.7 °F; 348.8 to 349.6 K)[2] | ||
| Boiling point | 323.9 °C (615.0 °F; 597.0 K) at 760mmHg | ||
| Hazards | |||
| Flash point | 149.7 °C (301.5 °F; 422.8 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Asparagusic acid is an organosulfur compound with the molecular formula C4H6O2S2 and systematically named 1,2-dithiolane-4-carboxylic acid. The molecule consists of a heterocyclic disulfide functional group (a 1,2-dithiolane) with a carboxylic acid side chain. It is found in asparagus and is believed to be the metabolic precursor to odorous sulfur compounds responsible for the distinctive smell of urine which has long been associated with eating asparagus.[3][4]
Isolation and biosynthesis
The material was originally isolated from an aqueous extract of Asparagus officinalis, a spring vegetable.[5] It is a derivative of the cyclic disulfide organic compound 1,2-dithiolane with a carboxylic acid functional group bound to carbon-4 of the heterocycle. Biosynthetic studies revealed that asparagusic acid is derived from isobutyric acid.[6] Asparagusic acid is a colorless solid with a melting point of 75.7–76.5 °C,[2] higher than that of the corresponding dithiol: dihydroasparagusic acid (or γ,γ-dimercaptoisobutyric acid), at 59.5–60.5 °C.[7]
Laboratory synthesis
A convenient synthesis of asparagusic acid has been developed from a commercially available diethyl malonate derivative starting material, improving on the prior method of Jansen.[5] Diethyl bis(hydroxymethyl)malonate is treated with hydroiodic acid to yield β,β'-diiodoisobutyric acid after decarboxylation and ester hydrolysis (with removal of volatile ethanol and carbon dioxide). Dihydroasparagusic acid, the reduced (dithiol) form of asparagusic acid, is produced by sequential reaction with sodium trithiocarbonate (Na2CS3) and sulfuric acid; subsequent oxidation with hot dimethyl sulfoxide yields asparagusic acid.[1]
Effect on urine
Observations that eating asparagus results in a detectable change in the odour of urine have been recorded over time. In 1702, Louis Lémery noted "a powerful and disagreeable smell in the urine",[8] whilst John Arbuthnot noted that "asparagus ... affects the urine with a foetid smell."[9][10] Benjamin Franklin described the odour as "disagreable",[11] whilst Marcel Proust claimed that asparagus "transforms my chamber-pot into a flask of perfume."[10][12] As early as 1891, Marceli Nencki had attributed the smell to methanethiol.[13][14] The odour is attributed to a mixture of sulfur-containing metabolites of asparagusic acid.[3][4][15][16]
The origin of asparagus urine is asparagusic acid, a substance unique to this vegetable.[17][18] Most studies of the compounds responsible for the odour of asparagus urine have correlated the appearance of the compounds above with asparagus consumption; they appear as little as 15 minutes after consumption.[10] However, this does not provide information on the biochemical processes that lead to their formation.
Asparagusic acid and lipoic acid are similar in that both possess a 1,2-dithiolane ring with a carboxylic acid tethered to it; indeed, it has been reported that asparagusic acid can substitute for lipoic acid in α-keto-acid oxidation systems such as the citric acid cycle.[18] The (R)-(+)-enantiomer of α-lipoic acid is a cofactor in the pyruvate dehydrogenase complex and is essential for aerobic metabolism. The degradation pathway of lipoic acid has been well studied and includes evidence of reduction of the disulfide bridge, S-methylation, and oxidation to produce sulfoxides.[19] Similar transformations of asparagusic acid would lead to metabolites like this detected in asparagus urine. Synthetic work has confirmed the relative ease of oxidation of asparagusic acid to yield S-oxides of the dithiolane ring.[1] The rate of degradation appears highly variably between subjects; the typical half-life for odour disappearance is around 4 h with a between subject variability of 43.4%.[20]
In the small minority of people who do not produce these metabolites after consuming asparagus, the reason may be as simple as asparagusic acid not being taken into the body from the digestive tract[3] or that these individuals metabolise it in such a way as to minimise the release of volatile sulfur-containing products.[10]
References
- ↑ 1.0 1.1 1.2 Yanagawa, H.; Kato, T.; Sagami, H.; Kitahara, Y. (1973). "Convenient procedure for the synthesis of asparagusic acids". Synthesis 1973 (10): 607–608. doi:10.1055/s-1973-22265.
- ↑ 2.0 2.1 Foss, O.; Tjomsland, O. (1958). "Crystal and molecular structure of 1,2-dithiolane-4-carboxylic acid". Acta Chemica Scandinavica 12: 1810–1818. doi:10.3891/acta.chem.scand.12-1810.
- ↑ 3.0 3.1 3.2 Mitchell, S. C. (2001). "Food idiosyncrasies: Beetroot and asparagus". Drug Metabolism and Disposition 29 (4): 539–543. PMID 11259347. http://dmd.aspetjournals.org/content/29/4/539.full.
- ↑ 4.0 4.1 Pelchat, M. L.; Bykowski, C.; Duke, F. F.; Reed, D. R. (2011). "Excretion and perception of a characteristic odor in urine after asparagus ingestion: A psychophysical and genetic study". Chemical Senses 36 (1): 9–17. doi:10.1093/chemse/bjq081. PMID 20876394.
- ↑ 5.0 5.1 Jansen, E. F. (1948). "The isolation and identification of 2,2'-dithiolisobutyric acid from asparagus". Journal of Biological Chemistry 176 (2): 657–664. doi:10.1016/S0021-9258(19)52681-3. PMID 18889921.
- ↑ Parry, R. J.; Mizusawa, A. E.; Chiu, I. C.; Naidu, M. V.; Ricciardone, M. (1985). "Biosynthesis of sulfur compounds. Investigations of the biosynthesis of asparagusic acid". Journal of the American Chemical Society 107 (8): 2512–2521. doi:10.1021/ja00294a051.
- ↑ Singh, R.; Whitesides, G. M. (1990). "Comparisons of rate constants for thiolate-disulfide interchange in water and in polar aprotic solvents using dynamic 1H NMR line shape analysis". Journal of the American Chemical Society 112 (3): 1190–1197. doi:10.1021/ja00159a046.
- ↑ McGee, H. (2007). On Food and Cooking: The Science and Lore of the Kitchen (Illustrated ed.). Simon and Schuster. p. 315. ISBN 9781416556374. https://books.google.com/books?id=bKVCtH4AjwgC&pg=PA315.
- ↑ Arbuthnot, J. (1735). An Essay Concerning the Nature of Aliments, and the Choice of Them, According to the Different Constitutions of Human Bodies: In which the Different Effects, Advantages, and Disadvantages of Animal and Vegetable Diet, are Explain'd (3rd ed.). London: Jacob Tonson. p. 64. https://archive.org/details/b30538221. "An Essay Concerning the Nature of Ailments."
- ↑ 10.0 10.1 10.2 10.3 Stromberg, J. (3 May 2013). "Why Asparagus Makes Your Urine Smell". smithsonian.com. http://www.smithsonianmag.com/science-nature/why-asparagus-makes-your-urine-smell-49961252/?no-ist. Retrieved 19 June 2016.
- ↑ Franklin, Benjamin (1781). Japikse, Carl. ed. Fart Proudly: Writings of Benjamin Franklin You Never Read in School (Revised ed.). ISBN 9780898048018. https://archive.org/details/fartproudlywriti0000fran.
- ↑ Proust, Marcel (1988) (in French). Du côté de chez Swann. Gallimard. "changer mon pot de chambre en un vase de parfum"
- ↑ Nencki, M. (1891). "Ueber das vorkommen von methylmercaptan im menschlichen harn nach spargelgenuss". Archiv für experimentelle Pathologie und Pharmakologie 28 (3): 206–209. doi:10.1007/BF01824333.
- ↑ Richer, C.; Decker, N.; Belin, J.; Imbs, J. L.; Montastruc, J. L.; Giudicelli, J. F. (1989). "Odorous urine in man after asparagus". British Journal of Clinical Pharmacology 27 (5): 640–641. doi:10.1111/j.1365-2125.1989.tb03431.x. PMID 2757887.
- ↑ White, R. H. (1975). "Occurrence of S-methyl thioesters in urines of humans after they have eaten asparagus". Science 189 (4205): 810–811. doi:10.1126/science.1162354. PMID 1162354. Bibcode: 1975Sci...189..810W.
- ↑ Waring, R. H.; Mitchell, S. C.; Fenwick, G. R. (1987). "The chemical nature of the urinary odour produced by man after asparagus ingestion". Xenobiotica 17 (11): 1363–1371. doi:10.3109/00498258709047166. PMID 3433805.
- ↑ Mitchell, S. C. (2013). "Asparagus, urinary odor, and 1,2-dithiolane-4-carboxylic acid". Perspectives in Biology and Medicine 56 (3): 341–351. doi:10.1353/pbm.2013.0031. PMID 24375116.
- ↑ 18.0 18.1 Mitchell, S. C.; Waring, R. H. (2014). "Asparagusic acid". Phytochemistry 97: 5–10. doi:10.1016/j.phytochem.2013.09.014. PMID 24099657. Bibcode: 2014PChem..97....5M.
- ↑ Schupke, H.; Hempel, R.; Peter, G.; Hermann, R.; Wessel, K.; Engel, J.; Kronbach, T. (2001). "New metabolic pathways of α-lipoic acid". Drug Metabolism and Disposition 29 (6): 855–862. PMID 11353754. http://dmd.aspetjournals.org/content/29/6/855.long.
- ↑ van Hasselt, J. G. C.; Elassaiss-Schaap, J.; Ramamoorthy, A.; Sadler, B. M.; Kasichayanula, S.; Edwards, Y.; van der Graaf, P. H.; Zhang, L. et al. (2016). "The proof is in the pee: Population asparagus urinary odor kinetics". PAGE: Abstracts of the Annual Meeting of the Population Approach Group in Europe 25. ISSN 1871-6032. http://www.page-meeting.org/default.asp?abstract=5717. Retrieved 7 July 2016.
External links
 |